
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21, Problem 30P
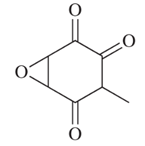
Terreic acid, a naturally occurring antibiotic substance, is an enol isomer of the structure shown. Write the two most stable enols and choose the more stable one.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Explain the general reaction of enolates—reaction with other carbonyl compounds ?
Tranexamic acid, a drug useful against blood clotting, is prepared commercially from p-methylbenzonitrile. Following is one of the steps in its synthesis, draw the structure of the product of this step.
Identify compounds A – E of the reaction sequence shown in Scheme III, making sure to include stereochemistry as appropriate.a) Identify compound A in Scheme III.
b) Identify compound B in Scheme III.
c) Identify compound C in Scheme III.
d) Identify compound D in Scheme III.
e) Identify compound E in Scheme III.
Chapter 21 Solutions
Organic Chemistry - Standalone book
Ch. 21.1 - Prob. 1PCh. 21.1 - Prob. 2PCh. 21.1 - Prob. 3PCh. 21.1 - Prob. 4PCh. 21.1 - Prob. 5PCh. 21.2 - Prob. 6PCh. 21.2 - Prob. 7PCh. 21.2 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.3 - Prob. 10P
Ch. 21.3 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - Problem 21.15 Write the structure of the Dieckmann...Ch. 21.5 - Prob. 16PCh. 21.5 - Prob. 17PCh. 21.6 - Prob. 18PCh. 21.6 - Prob. 19PCh. 21.6 - Prob. 20PCh. 21.6 - Prob. 21PCh. 21.6 - Prob. 22PCh. 21.7 - Prob. 23PCh. 21.8 - Problem 21.24 Mesityl oxide is an industrial...Ch. 21.8 - Prob. 25PCh. 21.8 - Prob. 26PCh. 21.8 - Prob. 27PCh. 21.8 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Terreic acid, a naturally occurring antibiotic...Ch. 21 - Prob. 31PCh. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - Give the structure of the expected organic product...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Give the structure of the principal organic...Ch. 21 - Prob. 41PCh. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - Prob. 44PCh. 21 - Prob. 45PCh. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - The use of epoxides as alkylating agents for...Ch. 21 - Prob. 49PCh. 21 - Show how you could prepare each of the following...Ch. 21 - Prob. 51PCh. 21 - Prob. 52PCh. 21 - Prob. 53PCh. 21 - Prob. 54PCh. 21 - The - methylene ketone sarkomycin has an...Ch. 21 - - Lactone can be prepared in good yield from...Ch. 21 - Prob. 57PCh. 21 - Prob. 58DSPCh. 21 - The Enolate Chemistry of Dianionss The synthetic...Ch. 21 - Prob. 60DSPCh. 21 - Prob. 61DSPCh. 21 - Prob. 62DSPCh. 21 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Nicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forwardA key step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involved the reaction of compound A and dihalide B with two equivalents of LDA to form C. Draw a stepwise mechanism for this reaction. β-Vetivone contains a spiro ring system—that is, two rings that share a single carbon atom.arrow_forwardWhat product is obtained when the following vicinal diol is heated in an acidic solution?arrow_forward
- What is the major organic product obtained from the following reaction under low temperature conditions? A, B, C, or D?arrow_forwardFrom what you have learned about enols and the hydration of alkynes, predict what product is formed by the acid-catalyzed hydration of CH3CH2CH2C = COCH3. Draw a stepwise mechanism that illustrates how it is formed.arrow_forwardAnswer the following question about curcumin, a yellow pigmentisolated from turmeric, a tropical perennial in the ginger family and aprincipal ingredient in curry powder. Most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols.arrow_forward
- 3. Draw the structure of the main organic product of the following reactions.arrow_forwardExplain why the following bromoketone forms different bicyclic compounds under different reaction conditions:arrow_forwardExplain the reactivity and orientation effects observed in each heterocycle.a. Pyridine is less reactive than benzene in electrophilic aromatic substitution and yields 3-substituted products.b. Pyrrole is more reactive than benzene in electrophilic aromatic substitution and yields 2-substituted products.arrow_forward
- Explain the reactivity and orientation effects observed in each heterocycle. a. Pyridine is less reactive than benzene in electrophilic aromatic substitution and yields 3-substituted products. b. Pyrrole is more reactive than benzene in electrophilic aromatic substitution and yields 2-substituted products.arrow_forwardAnswer the following questions about curcumin, a yellow pigment isolated from turmeric, a tropical perennial in the ginger family and a principal ingredient in curry powder.a.In Chapter 11, we learned that most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols. b.Explain why the enol O—H proton is more acidic than an alcohol O—H proton. c. Why is curcumin colored? d.Explain why curcumin is an antioxidant.arrow_forwardComplete the following reaction scheme. Draw the structure for compound A. Be sure to show stereochemistry and do not include any byproducts. Draw the structure for compound B. Be sure to show stereochemistry and do not include any byproducts.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License