
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.8, Problem 24P
Problem 21.24
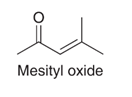
Mesityl oxide is an industrial chemical prepared by an aldol condensation. From what organic starting material is mesityl oxide prepared? It often contains about
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Devise a synthesis of optically active (S)-fluoxetine (trade name Prozac) from the given starting materials and any other needed reagents.
β-Vetivone is isolated from vetiver, a perennial grass that yields a variety of compounds used in traditional eastern medicine, pest control, and fragrance. In one synthesis, ketone A is converted to β-vetivone by a two-step process:Michael reaction, followed by intramolecular aldol reaction. (a) What Michael acceptor is needed for the conjugate addition? (See Problem 23.61 for another method to form the bicyclic ring system of β-vetivone.) (b) Draw a stepwise mechanism for the aldol reaction, which forms the six-membered ring.
Carboxylic acid X is an intermediate in the multistep synthesis of proparacaine, a local anesthetic. Devise a synthesis of X from phenol and any needed organic or inorganic reagents.
Chapter 21 Solutions
Organic Chemistry - Standalone book
Ch. 21.1 - Prob. 1PCh. 21.1 - Prob. 2PCh. 21.1 - Prob. 3PCh. 21.1 - Prob. 4PCh. 21.1 - Prob. 5PCh. 21.2 - Prob. 6PCh. 21.2 - Prob. 7PCh. 21.2 - Prob. 8PCh. 21.3 - Prob. 9PCh. 21.3 - Prob. 10P
Ch. 21.3 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - Problem 21.15 Write the structure of the Dieckmann...Ch. 21.5 - Prob. 16PCh. 21.5 - Prob. 17PCh. 21.6 - Prob. 18PCh. 21.6 - Prob. 19PCh. 21.6 - Prob. 20PCh. 21.6 - Prob. 21PCh. 21.6 - Prob. 22PCh. 21.7 - Prob. 23PCh. 21.8 - Problem 21.24 Mesityl oxide is an industrial...Ch. 21.8 - Prob. 25PCh. 21.8 - Prob. 26PCh. 21.8 - Prob. 27PCh. 21.8 - Prob. 28PCh. 21 - Prob. 29PCh. 21 - Terreic acid, a naturally occurring antibiotic...Ch. 21 - Prob. 31PCh. 21 - Prob. 32PCh. 21 - Prob. 33PCh. 21 - Prob. 34PCh. 21 - Give the structure of the expected organic product...Ch. 21 - Prob. 36PCh. 21 - Prob. 37PCh. 21 - Prob. 38PCh. 21 - Prob. 39PCh. 21 - Give the structure of the principal organic...Ch. 21 - Prob. 41PCh. 21 - Prob. 42PCh. 21 - Prob. 43PCh. 21 - Prob. 44PCh. 21 - Prob. 45PCh. 21 - Prob. 46PCh. 21 - Prob. 47PCh. 21 - The use of epoxides as alkylating agents for...Ch. 21 - Prob. 49PCh. 21 - Show how you could prepare each of the following...Ch. 21 - Prob. 51PCh. 21 - Prob. 52PCh. 21 - Prob. 53PCh. 21 - Prob. 54PCh. 21 - The - methylene ketone sarkomycin has an...Ch. 21 - - Lactone can be prepared in good yield from...Ch. 21 - Prob. 57PCh. 21 - Prob. 58DSPCh. 21 - The Enolate Chemistry of Dianionss The synthetic...Ch. 21 - Prob. 60DSPCh. 21 - Prob. 61DSPCh. 21 - Prob. 62DSPCh. 21 - Prob. 63DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Devise a synthesis of compound A from the given starting materials. Youmay use any other inorganic reagents or organic alcohols. A was used toprepare aliskiren, a drug used to treat hypertensionarrow_forwardThe analgesic naproxen can be prepared by a stepwise reaction sequence from ester A. Using enolate alkylation in one step, what reagents are needed to convert A to naproxen? Draw the structure of each intermediate. Explain why a racemic product is formed.arrow_forwardBupropion, sold under the trade name of Zyban, is an antidepressant that was approved to aid smoking cessation in 1997. Devise a synthesis of bupropion from benzene, organic compounds that have fewer than ve carbons, and any required inorganic reagents.arrow_forward
- Devise a synthesis of A from the three starting materials given. You may use any other needed organic or inorganic reagents.arrow_forward(−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from Atropa belladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated. (a) Explain this result by drawing a stepwise mechanism. (b) Explain why littorine, an isomer isolated from the tailflower plant in Australia, can be obtained optically pure regardless of the amount of base used during isolation.arrow_forwardWhat product is formed when a solution of A and B is treated with mild base? This reaction is the rst step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Introduction to Organometallic Compounds; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=3FRV31YYtL8;License: Standard YouTube License, CC-BY