
Concept explainers
Compound W was isolated from a marine annelid commonly used in Japan as a fish bait, and it was shown to be the substance that gives this organism its observed toxicity to some insects that contact it.
MS (m/z): 151 (relative abundance 0.09), 149 (

IR (cm−1): 2960, 2850, 2775
1H NMR (δ): 2.3 (s, 6H), 2.6 (d, 4H), and 3.2 (pentet, 1H)
13C NMR (δ): 38 (CH3), 43 (CH2), and 75 (CH)
These reactions were used to obtain further information about the structure ofW:
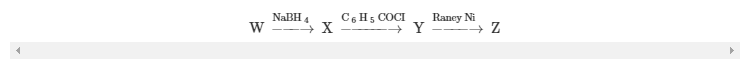
Compound X had a new infrared band at 2570 cm−1and these NMR data:
1H NMR(δ): 1.6 (t, 2H), 2.3 (s, 6H), 2.6 (m, 4H), and 3.2 (pentet, 1H)
13C NMR(δ): 28 (CH2), 38 (CH3), and 70 (CH)
Compound Y had these data:
IR (cm−1): 3050, 2960, 2850, 1700, 1610, 1500, 760, 690
1H NMR (δ): 2.3 (s, 6H), 2.9 (d, 4H), 3.0 (pentet, 1H), 7.4 (m, 4H), 7.6 (m, 2H), and 8.0 (m, 4H)
13C NMR (δ): 34 (CH2), 39 (CH3), 61 (CH), 128 (CH), 129 (CH), 134 (CH), 135 (C), and 187 (C)
Compound Z had
MS (m/z): 87 (

IR (cm−1): 2960, 2850, 1385, 1370, 1170
1H NMR (δ): 1.0 (d, 6H), 2.3 (s, 6H), and 3.0 (heptet, 1H)
13C NMR (δ): 21 (CH3), 39 (CH3), and 55 (CH)
What are the structures of W and of each of its reaction products X, Y, and Z?
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Organic Chemistry (Looseleaf) (Custom Package)
Additional Science Textbook Solutions
CHEMISTRY-TEXT
Chemistry: Matter and Change
Chemistry: The Central Science (14th Edition)
Organic Chemistry As a Second Language: Second Semester Topics
Chemistry: The Central Science (13th Edition)
Elementary Principles of Chemical Processes, Binder Ready Version
- In 250 ml ethanol solution, a tablet of saccharin was dissolved, and it was measured by UV spectrometer at a wavelength of 310 nm. The absorption was 0.45, the cell length was 1 cm, and the molar absorptivity was 12500. Calculate: the concentration of saccharin in the solution?arrow_forwardStructure pK, K. Name NH; CHCH,-O 2.20 (СО,Н) 9.31 (NH,) 6.3 X 10-3 4.9 x 10-10 Phenylalanine CO,H 7.11 x 10-3 6.34 x 10-8 2.148 * 7.198 Phosphoric acid* (hydrogen phosphate) НО — Р— Он 12.375 4.22 X 10-13 OH (1.5) 6.78 3 x 10-2 1.66 x 10-7 HP- он Phosphorous acid (hydrogen phosphite) OH 1.12 x 10-3 3.90 x 10-6 2.950 Phthalic acid (benzene-1,2-dicarboxylic acid) 5.408 cO,H Piperazine (perhydro- 1,4-diazine) 5.333 9.731 4.65 x 10-6 1.86 X 10-10 11.125 7.50 x 10-12 Piperidine 1.12 x 10-2 2.29 x 10-11 -co,H 1.952 (СO,Н) 10.640 (NH,) Prolinc +H 4.874 1.34 x 10-5 Propanoic acid CH,CH,CO,H H,C=CHCO,H 4.258 5.52 x 10-5 Propenoic acid (аcrylic acid) 10.566 2.72 x 10-11 Propylamine CH;CH,CH,NII; 5.20 6.3,X 10-6 Pyridine (azine) Pyridine-2-carboxylic acid (picolinic acid) (1.01) (CO,H) 5.39 (NH) 9.8 x 10-2 4.1 x 10-6 co,H HO,C Pyridine-3-carboxylic acid (nicotinic acid) 2.03 (CO,H) 4.82 (NH) 9.3 x 10-3 1.51 x 10-s NH+ 1.4 (POH) (u = 0.1) 3.44 (OH) (1p. = 0.1) 6.01 (POH) (u = 0.1) 8.45 (NH) (u =…arrow_forward6. (Chapter 15 - 47b) An aromatic compound, C9H12 has the following H NMR spectrum and a peak at 750 cmt in its IR spectrum. Answer the following questions. Chem. shift Rel. area 1.19 2.31 2.64 7.13 1.50 1.50 1.00 2.00 TMS 10 3 O ppm Chemical shift (6) 4(a). The IR peak at 750 cm in its IR spectrum indicate that the compound is. disubstituted = 4(b) The name of the compound is = Intensity-arrow_forward
- Following is a retrosynthetic analysis for the anthelmintic (against worms) diethylcarbamazine. N. `NET2 N. OEt Me Me Diethylcarbamazine (A) (2) OH MENH, CI OEt Me OH Me Methylamine Ethylene (C) (В) Ethyl oxide chloroformate Diethylcarbamazine is used chiefly against nematodes, small cylindrical or slender threadlike worms such as the common roundworm, which are parasitic in animals and plants. Given this retrosynthetic analysis, propose a synthesis of diethylcarbamazine from the three named starting materials.arrow_forwardUV: max 235 (ε 12,000) and max 314 (ε 120) EIMS: m/z 39 (31%), 41 (33%), 55 (37%), 69 (26%), 98 (100%), 99 (7%), 100 (0.2%) IR: Imm IR spectrum in CCl4 solvent 2950 cm-¹ 2000 4) RC=N 5) 0-H BAYEHUNIERI - R 1630 cm-¹ 1710 cm-¹ $10 4a) Which of the following heteroatoms that are definitely NOT present based on the EIMS data? (Circle all that apply). Br Cl FSI 0 4 continued) Please refer to data on preceding page. 4b) From the EIMS data what is the best estimate of the number of carbon atoms in this molecule? 4c) From the UV data, conjugated л-bonds are: definitely present definitely absent 4d) From the IR spectrum alone, which of the following functional groups can be definitely ruled OUT? Circle those that are ruled out and briefly explain why. 1) C=0 2) RC=CR 3) C=C cannot tellarrow_forwardA stock solution of alcohol dehydrogenase (ADH) has been diluted 1 in 10 and the absorbance reading at 280 nm of this diluted solution is 0.04. Given that the absorption coefficient for ADH at 280 nm is e mg/ml = 1.6 and that you require to do 30 assays in triplicate, each assay using 2 ug of enzyme, how many ul of the stock ADH do you need to do the experiment?arrow_forward
- Most of the pKa values given in this text were determined in water. How would the pKa values of carboxylic acids, alcohols, ammonium ions (RNH32) , phenol, and an anilinium ion (C6H5NH32) change if they were determined in a solvent less polar than water?arrow_forwardO 11:E9 5310107675680311588_2 50.What is the pH of a 0.10 M solution of trimethylamine (pKs = 4.13)? 17 51.How would you calculate K, for the formate ion, given that the K, for formic acid is 1.8 x 10-4? (Kw=1.0x 10-14) K = K, x Kw b. K = Kw/K, c. K, = K/K d. K = Kw + Ka e. K = Kw - K, a. IIarrow_forwardCa(QCI)2 + 2 CH3COOH E-> 2HOCI + (CH3COO)2Ca|arrow_forward
- Chemistry Estimation of Rhodamine B in Wastewater-UV-VIS Spectrophotometryarrow_forwardCompound 47 is a high-boiling liquid (boiling point 204-205 'C), that reacts with Ag* ion in aqueous ammonia to form a "silver mirror" (the Tollens test). The Mass, IR, and 'H NMR spectra, along with 13C NMR data, are given below. Elemental Analysis: C, 79.97; H, 6.71; O, 13.32.arrow_forwardIn your job as a chemist for a regional analytical laboratory, you are expecting many samples that you will need to test for potential herbicide contamination. Explain, which type of detectors you will need for the trace-level GC analysis of (a) 2,4-Dichlorophenoxyacetic acid and (b) atrazine. ÇI N° ОН 'N' N' CI CI a b ZIarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
