
Concept explainers
(a)
Interpretation: The products resulting from the vigorous oxidation of the given compound by
Concept Introduction:
Oxidation with
The carbon atom attached to the benzene ring is known as benzylic carbon atom. The benzylic carbon that is attached with a benzylic hydrogen atom will be oxidized into carboxylic acid group on treatment with chromic acid
General scheme:
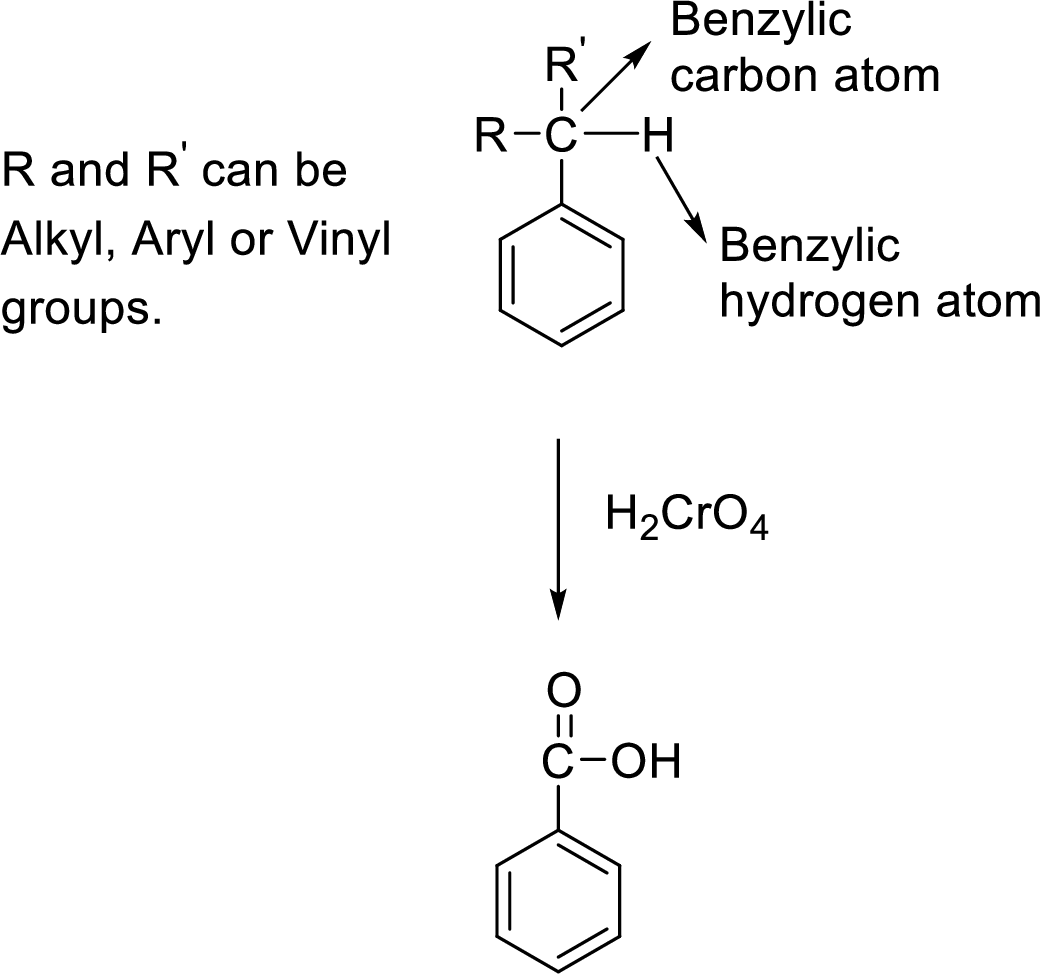
(b)
Interpretation: The products resulting from the vigorous oxidation of the given compound by
Concept Introduction:
Oxidation with
The carbon atom attached to the benzene ring is known as benzylic carbon atom. The benzylic carbon that is attached with a benzylic hydrogen atom will be oxidized into carboxylic acid group on treatment with chromic acid
General scheme:
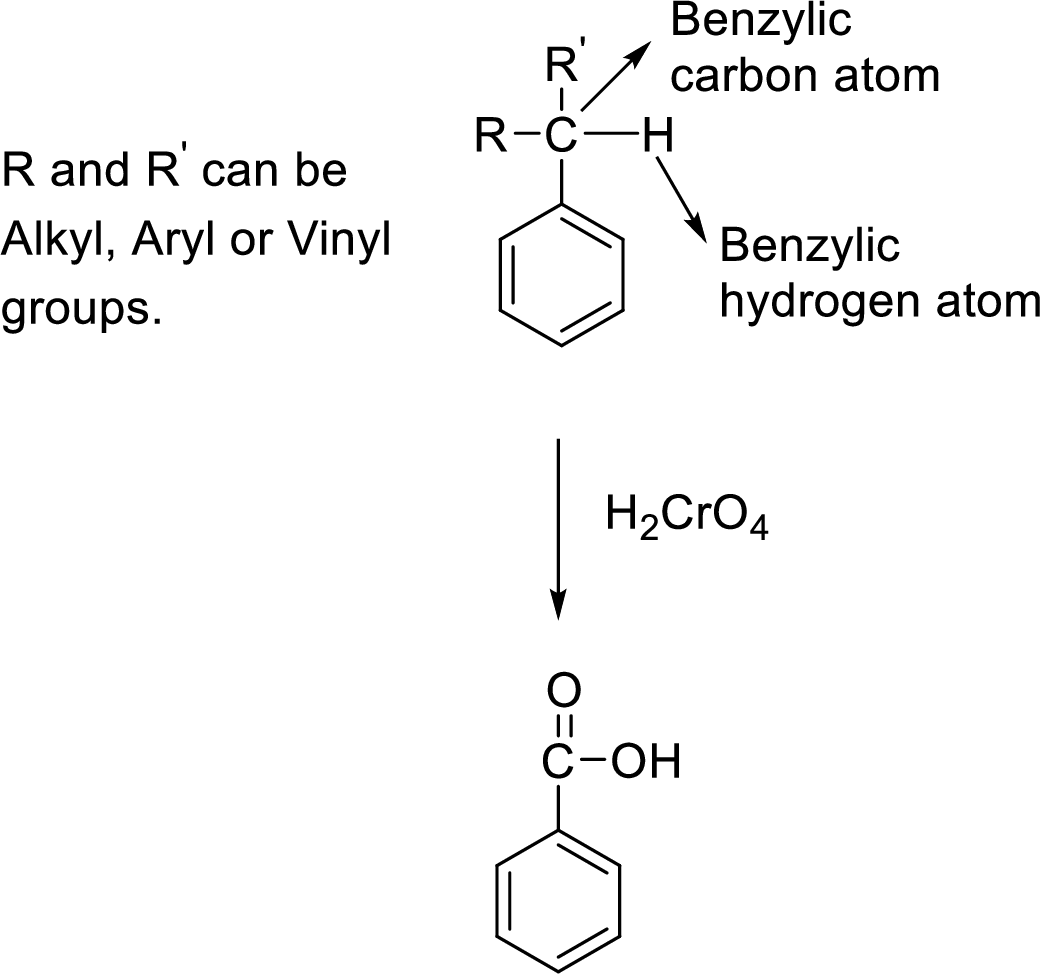
In this oxidation, halogen and nitro substituents on the benzene ring are unaffected as shown in the following example:
Example:
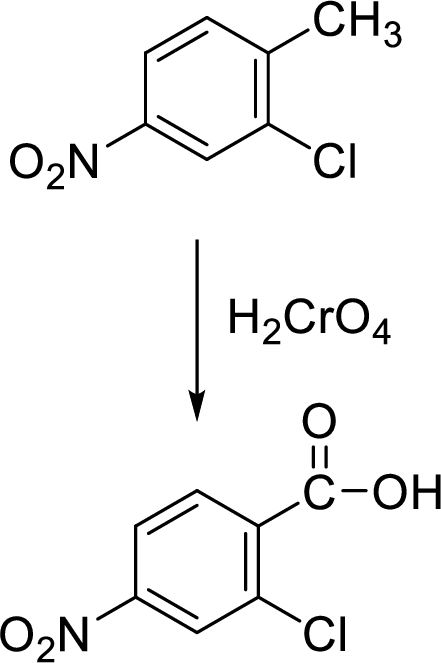
Trending nowThis is a popular solution!

Chapter 21 Solutions
Organic Chemistry
- What product will be made with aqueous acid?arrow_forwardDraw balanced chemical equations for the Birch reduction of the following benzene derivatives. Please explain steps 1a)Anisole 1b) Aniline 1c) Phenol 1d) Toluenearrow_forwardWhat is the products you expect from oxidation of 2-Hexanol with CrO3 and H3O+?arrow_forward
- Choose the best reagents from the list provided below for carrying out the following conversion. Match the reagent with the step number. HCl (aq), Zn(Hg) Br2, FeBr3 Na/NH3, -33 degrees C NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heatarrow_forwardPredict the major product when each reagent reacts with ethylene oxide.(a) NaOCH2CH3 (sodium ethoxide)arrow_forward(please answer all questions) 1) Predict the products from reaction of 1-hexyne with thesereagents: a) One equivalent HBr b) One equivalent Cl2 c) H2 , Lindar Catalyst d) NaNH2 in NH3 , then CH3Brarrow_forward
- Choose the appropriate reagent for the following reaction. A) TsCl/pyridine B) SOCl2/pyridine C) ZnCl2 D) PBr3 E) HIarrow_forwardPredict the major product when each reagent reacts with ethylene oxide a. NaSPh (sodium thiophenoxide) b. PhNH2 (aniline)arrow_forwardProvide the reagents necessary to complete the following reactions. More than one step may be necessary, if so number separate steps.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
