
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 21.SE, Problem 68AP
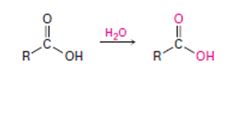
When a carboxylic acid is dissolved in isotopically labeled water, the label rapidly becomes incorporated into both oxygen atoms of the carboxylic acid. Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Explain why pentane-2,4-dione forms two alkylation products (A and B) when the number ofequivalents of base is increased from one to two.
Select the stronger acid from pair and explain your reasoning. For stronger acid, write a structural formula for its conjugate base.
Q.CH3CH2OH or CH3CH2SH
Rank the compounds from most to least acidic
Chapter 21 Solutions
Organic Chemistry
Ch. 21.1 - Give IUPAC names for the following substances:Ch. 21.1 - Draw structures corresponding to the following...Ch. 21.2 - Prob. 3PCh. 21.2 - Rank the compounds in each of the following sets...Ch. 21.2 - Predict the products of the following nucleophilic...Ch. 21.2 - Prob. 6PCh. 21.3 - Prob. 7PCh. 21.3 - If the following molecule is treated with acid...Ch. 21.4 - How might you prepare the following esters using a...Ch. 21.4 - Prob. 10P
Ch. 21.4 - Prob. 11PCh. 21.4 - Prob. 12PCh. 21.4 - Prob. 13PCh. 21.5 - Prob. 14PCh. 21.5 - What product would you expect from reaction of one...Ch. 21.6 - Prob. 16PCh. 21.6 - Prob. 17PCh. 21.6 - Show the products you would obtain by reduction of...Ch. 21.6 - What ester and what Grignard reagent might you...Ch. 21.7 - Prob. 20PCh. 21.7 - How would you use the reaction of an amide with...Ch. 21.8 - Write the mechanism of the reaction shown in...Ch. 21.9 - Prob. 23PCh. 21.9 - Prob. 24PCh. 21.10 - Prob. 25PCh. 21.10 - Prob. 26PCh. 21.SE - Name the following compounds:Ch. 21.SE - Prob. 28VCCh. 21.SE - Prob. 29VCCh. 21.SE - Prob. 30VCCh. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the mechanism...Ch. 21.SE - Predict the product(s) and provide the complete...Ch. 21.SE - Prob. 35MPCh. 21.SE - When 4-dimethylaminopyridine (DMAP) is added in...Ch. 21.SE - Prob. 37MPCh. 21.SE - Prob. 38MPCh. 21.SE - Prob. 39MPCh. 21.SE - The hydrolysis of a biological thioester to the...Ch. 21.SE - Prob. 41MPCh. 21.SE - Prob. 42MPCh. 21.SE - Prob. 43MPCh. 21.SE - In the iodoform reaction, a triiodomethyl ketone...Ch. 21.SE - Give IUPAC names for the following compounds:Ch. 21.SE - Prob. 46APCh. 21.SE - Draw and name compounds that meet the following...Ch. 21.SE - Predict the product, if any, of reaction between...Ch. 21.SE - Prob. 49APCh. 21.SE - Prob. 50APCh. 21.SE - What product would you expect to obtain from...Ch. 21.SE - Prob. 52APCh. 21.SE - Prob. 53APCh. 21.SE - The following reactivity order has been found for...Ch. 21.SE - Prob. 55APCh. 21.SE - Outline methods for the preparation of...Ch. 21.SE - Prob. 57APCh. 21.SE - When ethyl benzoate is heated in methanol...Ch. 21.SE - tert-Butoxycarbonyl azide, a reagent used in...Ch. 21.SE - Prob. 60APCh. 21.SE - Prob. 61APCh. 21.SE - What is the structure of the polymer produced by...Ch. 21.SE - Polyimides with the structure shown are used as...Ch. 21.SE - Prob. 64APCh. 21.SE - Propose a structure for a compound, C4H7ClO2, that...Ch. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Prob. 67APCh. 21.SE - When a carboxylic acid is dissolved in...Ch. 21.SE - Prob. 69APCh. 21.SE - Prob. 70APCh. 21.SE - Prob. 71APCh. 21.SE - Phenyl 4-aminosalicylate is a drug used in the...Ch. 21.SE - N,N-Diethyl-m-toluamide (DEET) is the active...Ch. 21.SE - Tranexamic acid, a drug useful against blood...Ch. 21.SE - One frequently used method for preparing methyl...Ch. 21.SE - Prob. 76APCh. 21.SE - Assign structures to compounds with the following...Ch. 21.SE - Propose structures for compounds with the...Ch. 21.SE - Propose a structure for the compound with the...Ch. 21.SE - Draw the structure of the compound that produced...Ch. 21.SE - Prob. 81APCh. 21.SE - Epoxy adhesives are prepared in two steps. SN2...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete each acid-base reaction and predict whether the position of equilibrium lies toward the left or toward the right. (a) CH3CCH+CH3CH2ONa+CH3CH3OH (b) CH3CCCH2CH2OH+Na+NH2NH3(l)arrow_forwardDraw the major organic structure in a basic solution.arrow_forwardProvide Typed solution : Compare and contrast the acidic/basic catalyzed hydrolysis pathways of an ester, provide a generic example of an acid & base canalyzed pathway of an ester. Comparing the two via a venn diagram.arrow_forward
- Explain why CH3CONH2 is a stronger acid and a weaker base than CH3CH2NH2.arrow_forwardNotice that the carboxylic acid is a jumping off point to get to other types of molecular connection, replacing the -H on the hydroxy part with another carbon group to get an________, replacing the -OH with a chlorine to get an________, replacing the -H on the hydroxyl again with a carbonyl related group to get an________, or replacing the OH with an amine to get an_________.arrow_forwardRank these benzoic acid species in order of decreasing acidity.arrow_forward
- How did Emil Bayer manage to make the bark of the Willow Tree that contained the active ingredient and precursor to aspirin LESS acidic via his organic chemistry reaction. Be specific, show by chemical reaction and circle all functional groups (parts of the molecule) in the reactants that were altered in the products (circle these too.arrow_forwardExplain and show step by step how did the answer or IUPAC name derived in letter b,f,h,k, and L. Please answer it all. This is about Carboxylic acid and its derivative.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY