
Concept explainers
(a)
Interpretation:
The product formed by the treatment of ethyl butanoate
Concept introduction:
The replacement or substitution of one functional group with another functional group in any
Answer to Problem 22.48P
No product is formed by the treatment of ethyl butanoate with
Explanation of Solution
Esters do not undergo reaction with
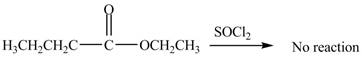
Figure 1
No product is formed by the treatment of ethyl butanoate with
(b)
Interpretation:
The product formed by the treatment of ethyl butanoate
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.48P
The product formed by the treatment of ethyl butanoate
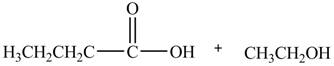
Explanation of Solution
Esters undergo hydrolysis under acidic and basic medium. In the given reaction, ester undergoes hydrolysis under acidic medium. One mole of carboxylic acid and one mole of alcohol are produced from the hydrolysis of ethyl butanoate as shown in Figure 2.

Figure 2
The product formed by the treatment of ethyl butanoate
(c)
Interpretation:
The product formed by the treatment of ethyl butanoate
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.48P
The product formed by the treatment of ethyl butanoate
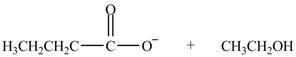
Explanation of Solution
Esters undergo hydrolysis under acidic and basic medium. In the given reaction, ester undergoes hydrolysis under basic medium. One mole of carboxylate ion and one mole of alcohol are produced from the hydrolysis of ethyl butanoate as shown in Figure 3.
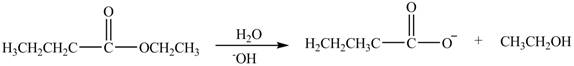
Figure 3
The product formed by the treatment of ethyl butanoate
(d)
Interpretation:
The product formed by the treatment of ethyl butanoate
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.48P
The product formed by the treatment of ethyl butanoate
![]()
Explanation of Solution
The reaction of ester with ammonia leads to the formation of amide and alcohol. The nitrogen atom of ammonia contains lone pair of electrons. The nitrogen atom acts as a nucleophile and attacks on the electron deficient carbonyl carbon atom of ester. The products formed by the reaction of ethyl butanoate with
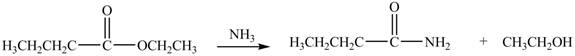
Figure 4
The product formed by the treatment of ethyl butanoate
(e)
Interpretation:
The product formed by the treatment of ethyl butanoate
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilic acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.48P
The product formed by the treatment of ethyl butanoate
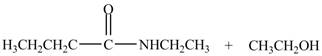
Explanation of Solution
The reaction of ester with primary
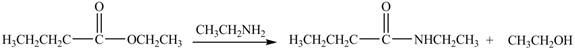
Figure 5
The product formed by the treatment of ethyl butanoate
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry (Looseleaf) - With Access
- 1. O-hydroxybenzoic acid is a major product formed with phenol and which other reactant/s I-primary alcohol II-sodium hydroxide III-water IV-carbon dioxide A.I and III B. I and IV C. II and III D. II and IVarrow_forwardDraw the products formed when phenol(C6H5OH) is treated with each reagent. Give an explanation. d. (CH3CH2)2CHCOCl, AlCl3 j. product in (d), then NH2NH2, – OHarrow_forwardDraw the product formed when (CH3)2CHOH is treated with each reagent (a, b and c)arrow_forward
- What reagents should be used?arrow_forwardDraw the products when phenylacetic acid (C6H5CH2COOH) is treated with each reagent. With some reagents no reaction occurs: A)NaCl B)NH3(1 eqiv) C)1)CH2NH2, 2)CH3COClarrow_forward1. What are the characteristics of a positive tollens test for adehydes? What is the oxidizing agent in tollens solutions? 2. What is the characteristics of a positive Benedict's test for aldehydes? What is the oxidizing agent in Benedict's solution?arrow_forward
- what reagents are needed?arrow_forwardShow how to prepare each compound from 2-methyl- 1- propanol. a. 2- methylpropene b. 2- methyl- 2- propanol c. 2- methylpropanoic acid (CH3)2CHCOOHarrow_forwardDraw the products a. Pentyl butanoate reacts with water and NaOH b. Acetic anhydride reacts with ethanol c. 3-hexanone reacts with two equivalents of isopropyl alcohol d. Hexanal reacts with NaBH4 and CH3OHarrow_forward
- Draw the product formed when the alcohol cyclobutanol is dehydrated with H2SO4.arrow_forwardDraw the products formed when each alcohol is dehydrated with H 2SO 4. Use the Zaitsev rule to predict the major product when a mixture forms.arrow_forwardDraw the products formed when each alkene is treated with HCl.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
