
Concept explainers
Devise a synthesis of each labeled compound using
a. 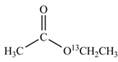 b.
b. 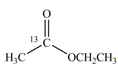 c.
c. 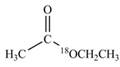 d.
d. 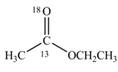
(a)
Interpretation:
The synthesis of given compound by the use of
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilc acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.75P
The synthesis of given compound by the use of
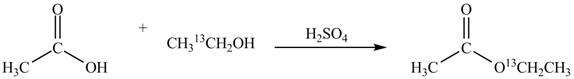
Explanation of Solution
An ester is formed by the reaction of carboxylic acid with alcohol. This reaction is known as Fischer Esterification. During this reaction, bond formation between carbon and oxygen takes place and water removes as a byproduct. In the synthesis of given compound, acetic acid is used as the source of unlabeled carbon atom and
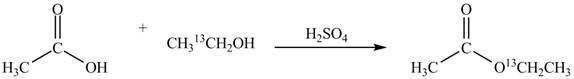
Figure 1
The synthesis of given compound by the use of
(b)
Interpretation:
The synthesis of given compound by the use of
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilc acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.75P
The synthesis of given compound by the use of
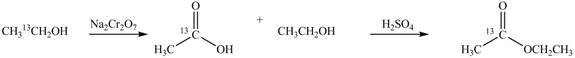
Explanation of Solution
An ester is formed by the reaction of carboxylic acid with alcohol. This reaction is known as Fischer Esterification. During this reaction, bond formation between carbon and oxygen takes place and water removes as a byproduct. In the synthesis of given compound,
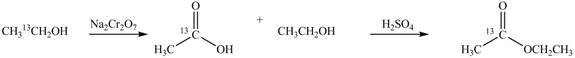
Figure 2
The synthesis of given compound by the use of
(c)
Interpretation:
The synthesis of given compound by the use of
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilc acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.75P
The synthesis of given compound by the use of
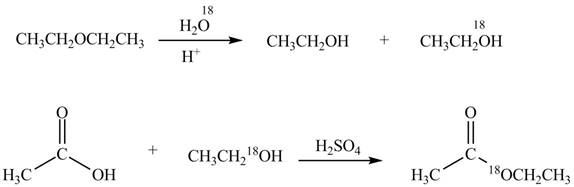
Explanation of Solution
An ester is formed by the reaction of carboxylic acid with alcohol. This reaction is known as Fischer Esterification. During this reaction, bond formation between carbon and oxygen takes place and water removes as a byproduct. In the synthesis of given compound, diethylether is first hydrolyzed to give

Figure 3
The synthesis of given compound by the use of
(d)
Interpretation:
The synthesis of given compound by the use of
Concept introduction:
The replacement or substitution of one functional group with another functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons is known as a nucleophile. In nucleophilc acyl substitution reaction, a nucleophile takes the position of a leaving group.
Answer to Problem 22.75P
The synthesis of given compound by the use of
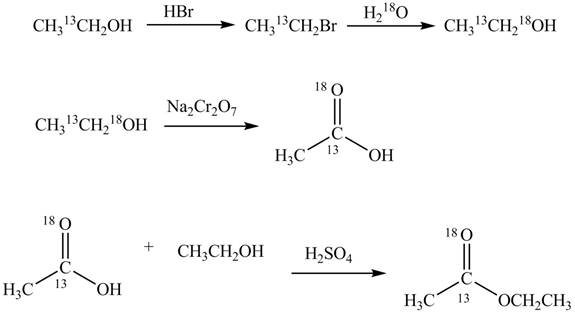
Explanation of Solution
An ester is formed by the reaction of carboxylic acid with alcohol. This reaction is known as Fischer Esterification. During this reaction, bond formation between carbon and oxygen takes place and water removes as a byproduct. In the synthesis of given compound,
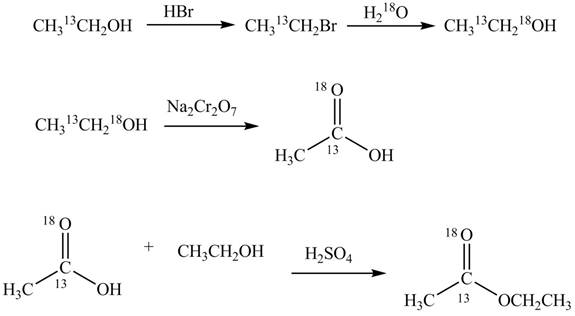
Figure 4
The synthesis of given compound by the use of
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry (Looseleaf) - With Access
- Devise a synthesis of each compound using CH3CH3 as the only sourceof carbon atoms. You may use any other required organic or inorganicreagents.arrow_forwardDevise a synthesis of each compound from benzene. You may also useany organic compounds having four or fewer carbons and any requiredinorganic reagents.arrow_forwardDevise a synthesis of each substituted cyclopropane. Use acetylene (HC=CH) as a starting material in part (a) and cyclohexanone as a starting material in part (b). You may use any other organic compounds and any needed reagents.arrow_forward
- (a) Give an acceptable name for compound A. (b) Draw the organic products formed when A is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forwardDevise a synthesis of each compound using CH3CH2CH=CH2 as the starting material. You may use any other organic compounds or inorganic reagents.arrow_forwardDevise a synthesis of each compound using 1-bromobutane (CH3CH2CH2CH2Br) as the only organic starting material. You may use any other inorganic reagents.arrow_forward
- Devise a synthesis of anastrozole, a drug used to reduce the recurrence of breast cancer (Section 22.18), from the given compounds. You may use any other needed organic compounds or inorganic reagents.arrow_forwardDevise a synthesis of anastrozole, a drug used to reduce the recurrence of breast cancer, from the given compounds. You may use any other needed organic compounds or inorganic reagents.arrow_forwardDevise a synthesis of benzocaine, ethyl p-aminobenzoate (H2NC6H4CO2CH2CH3), from benzene, organic alcohols, and any needed organic or inorganic reagents. Benzocaine is the active ingredient in the topical anesthetic Orajel (Section 18.15C).arrow_forward
- Devise a synthesis of each acetal from 1-bromo-2-methylhexane, alcohols (and diols) containing one or two carbons, and any needed inorganic reagents.arrow_forwardDevise a synthesis of each compound from cyclopentanone, benzene, and organic alcohols having ≤ 3 C's. You may also use any required organic or inorganic reagentsarrow_forwardDevise a synthesis of attached compound using CH3CH2CH=CH2 as the starting material. You may use any other organic compounds or inorganic reagents.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





