
(a)
Interpretation:
To identify the compound that has the most acidic proton in given set of compounds.
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton
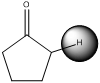
(b)
Interpretation:
To identify the compound that has the most acidic proton in given set of compounds.
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton
(c)
Interpretation:
To identify the compound that has the most acidic proton in given set of compounds.
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

(d)
Interpretation:
To identify the compound that has the most acidic proton in given set of compounds.
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

(e)
Interpretation:
To identify the compound that has the most acidic proton in given set of compounds.
Concept introduction:
pKa is negative base-10 logarithm of the dissociation constant of acid (Ka) of a solution.
pKa is used is to describe acid dissociation because it is expressed in small decimal numbers.
If pKa value is below 20 the compound is said to have acidic proton and vice versa
To identify : The compound pKa value and the most acidic proton

Want to see the full answer?
Check out a sample textbook solution
Chapter 22 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





