
Concept explainers
Interpretation:
The number of electros in the
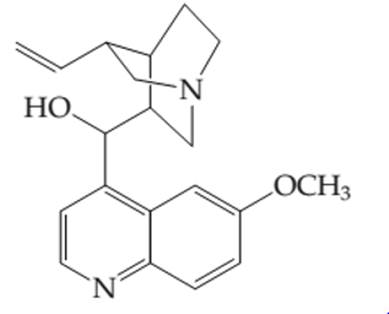
Concept introduction:
Organic compounds are the compounds which are mainly composed C and H atoms. The branch of chemistry that deals with preparation, reactions, and properties of organic compounds. The molecular formula of organic compound represents the number of bonded atoms with their atomic symbols.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY-MASTERINGCHEMISTRY W/ETEXT
- Please answer the below questionarrow_forwardDrug molecules Vicodin and Heroin, contain an amine functional group and several rings. Indicate the number of aromatic rings in each structure, and identify the amine functional group in each molecule as primary, secondary, or tertiary.arrow_forwardWhat molecule(s) are primarily responsible for the bitter taste of dark chocolate (the darkest chocolate is synonymous with bittersweet)?arrow_forward
- Please don't provide handwriting solutionarrow_forwardGive examples of aromatic compounds and non-aromatic compounds. Draw structures of each examplearrow_forwardHow come the structure labeled as 1 has 8 pi electrons? If the double bond has 2 pi electrons, and you count the lone pairs, wouldn't it have 6 pi electrons? Why does the structure labeled as 2 has aromaticity? If the pi bond has 2 pi electrons and then you coun the lone pairs, wouldn't it only have 5 pi electrons?arrow_forward
- Draw the structural formulas of compounds A, C, D, E and F in the boxes provided above.arrow_forward12) What is the major difference between an antiaromatic and aromatic compound? A) The structure must be cyclic for aromatic but not antiaromatic compounds? B) Antiaromatic compounds have at least one sp3 hybridized atom in the ring C) Antiaromatic compounds can assume a chair-like structure while aromatic compounds are nearly flat D) Aromatic compounds cannot have a charged atom in the structure E) Only aromatic compounds follow Huckle's rule.arrow_forwardCalicene (shown below) has an unusually large dipole. Draw an aromatic resonance structure that explains the dipole. Include all lone pairs in your structure.arrow_forward
- The parent among all aromatic compounds *arrow_forwardAnacin is an over-the-counter pain reliever that contains aspirin andcaffeine. Answer the following question about each compound mentioned in the attachment ? Question: Draw three additional resonance structures.arrow_forwardDefine Aromatic Compounds with More Than One Ring ?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





