
Concept explainers
(a)
Interpretation:
The synthesis of a given compound from
Concept introduction:
The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 23.70P
The formation of the given product from
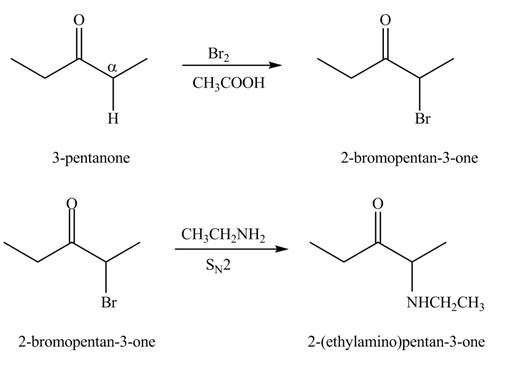
Explanation of Solution
A chemical equation for the given synthesis is shown below.
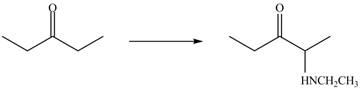
Figure 1
The formation of a given product by using
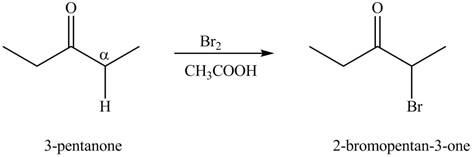
Figure 2
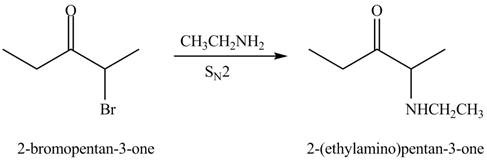
Figure 3
The first step of the reaction involves the bromination of
The formation of the given product from
(b)
Interpretation:
The synthesis of a given compound from
Concept introduction:
The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 23.70P
The synthesis of a given compound from
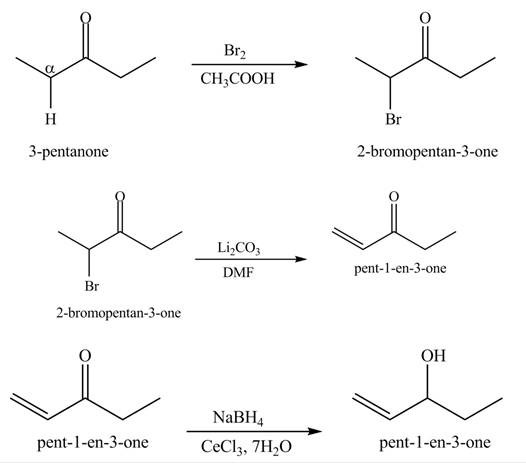
Explanation of Solution
A chemical equation for the given synthesis is shown below.

Figure 4
The formation of the given product by using
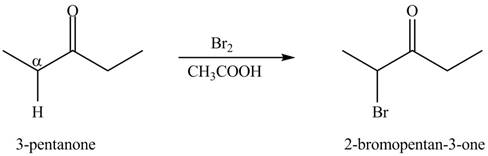
Figure 5
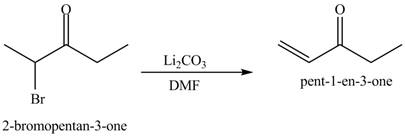
Figure 6
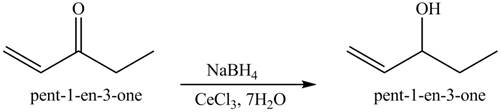
Figure 7
The first step of the reaction involves the bromination of
The synthesis of a given compound from
(c)
Interpretation:
The synthesis of a given compound from
Concept introduction:
The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 23.70P
The synthesis of a given compound from
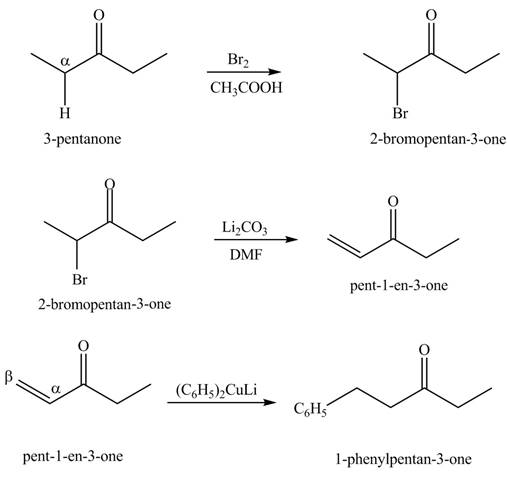
Explanation of Solution
A chemical equation for the given synthesis is shown below.
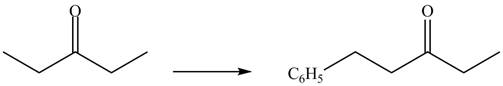
Figure 8
The formation of a given product by using
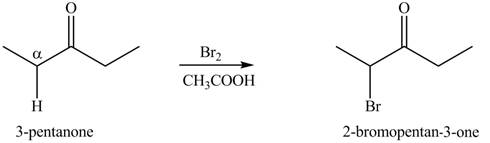
Figure 9
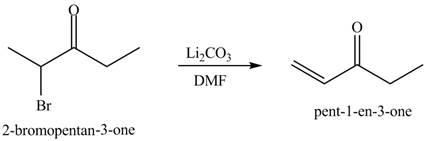
Figure 10
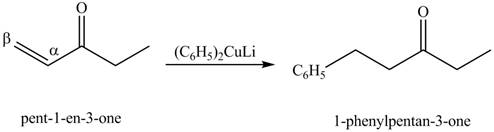
Figure 11
The first step of the reaction involves the bromination of
The synthesis of a given compound from
(d)
Interpretation:
The synthesis of a given compound from
Concept introduction:
The synthesis of the products relies upon the type of reactants and reagents that are used during the reactions. The energy of a target molecule should be low because it increases the stability of a molecule that results in the formation of a molecule with high yield. The reagents perform numerous functions in reactions like proton abstraction, oxidation, reduction, catalysis, and dehydrogenation.
Answer to Problem 23.70P
The synthesis of a given compound from
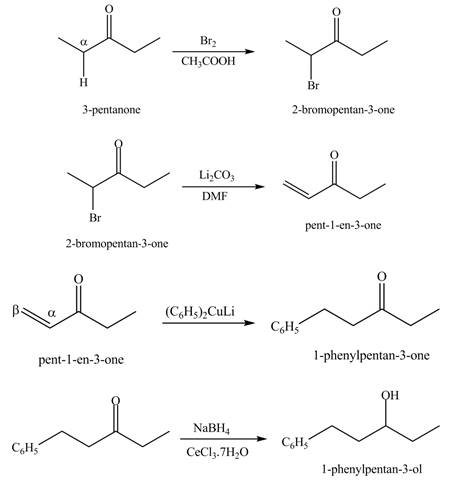
Explanation of Solution
A chemical equation for the given synthesis is shown below.
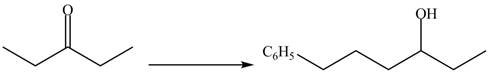
Figure 12
The formation of a given product by using
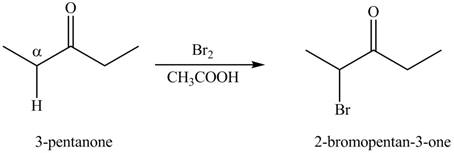
Figure 13
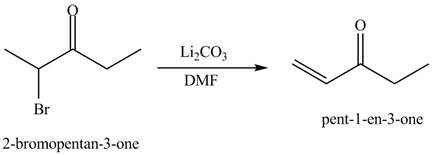
Figure 14
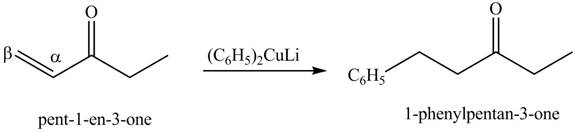
Figure 15
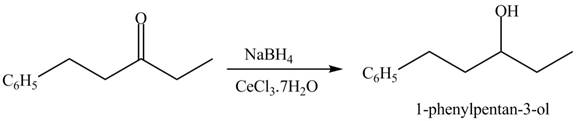
Figure 16
The first step of the reaction involves the bromination of
The synthesis of a given compound from
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Nicotinic acid, more commonly named niacin, is one of the B vitamins. Show how nicotinic acid can be converted to (a) ethyl nicotinate and then to (b) nicotinamide.arrow_forwardAnswer the following questions about curcumin, a yellow pigment isolated from turmeric, a tropical perennial in the ginger family and a principal ingredient in curry powder.a.In Chapter 11, we learned that most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols. b.Explain why the enol O—H proton is more acidic than an alcohol O—H proton. c. Why is curcumin colored? d.Explain why curcumin is an antioxidant.arrow_forwardProvide all needed reagents and conditions for each step.arrow_forward
- A. OsO4 and NMO B. Br2 and H20 C. Hg(OAc)2, H2O and NaBH4, NaOH D. RCO3H E. BH3-THF and H2O2, NaOH Which reagent will complete this reaction?arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs.a. NaBH4, CH3OHb. [1] LiAlH4; [2] H2Oc. [1] CH3MgBr (excess); [2] H2Od. [1] C6H5Li (excess); [2] H2Oe. Na2Cr2O7, H2SO4, H2Oarrow_forward(a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
- Answer the following question about curcumin, a yellow pigmentisolated from turmeric, a tropical perennial in the ginger family and aprincipal ingredient in curry powder. Most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols.arrow_forwardWhat alkyl halide and nucleophile are needed to prepare each compound?arrow_forward#20 B Draw structural formulas for all possible carbocations formed by the reaction of each alkene with HCl.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
