
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.SE, Problem 63AP
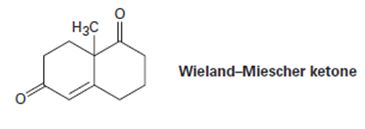
The so-called Wieland-Miescher ketone is a valuable starting material used in the synthesis of steroid hormones. How might you prepare it from 1, 3-cyclohexanedione?
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
A key step in a synthesis of the antimalarial drug quinine involves an intramolecular nucleophilic substitution that converts A to B. Draw the structure of B and give the reagents needed to convert B to quinine.
A key step in the synthesis of the narcotic analgesic meperidine (trade name Demerol) is the conversion of phenylacetonitrile to X. (a) What is the structure of X? (b) What reactions convert X to meperidine?
H3C-
H3C.
OCH 3
H3CO
CH3
H
Aldehydes and ketones react reversibly with two equivalents of alcohol in the presence of an acid catalyst to give acetals. Alcohols are poor nucleophiles, and so protonation of the carbonyl oxygen is
used to make the carbonyl carbon a stronger electrophile. Addition of the first equivalent of alcohol gives a hemiacetal, a hydroxyether. Addition of the second equivalent of alcohol is accompanied by
loss of water to yield the product acetal.
Draw curved arrows to show the movement of electrons in this step of the mechanism.
Arrow-pushing Instructions
NA
2 CH₂OH
HCI catalyst
CH3 HỘ
H3CQ
H3C-
H3CO
CH3
H3C.
OCH3
CH3
H₂O*:
45
Chapter 23 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 23.1 - Prob. 1PCh. 23.1 - Prob. 2PCh. 23.3 - Prob. 3PCh. 23.3 - Prob. 4PCh. 23.4 - Prob. 5PCh. 23.4 - 1-Butanol is prepared commercially by a route that...Ch. 23.4 - Prob. 7PCh. 23.5 - Prob. 8PCh. 23.6 - Prob. 9PCh. 23.6 - What product would you Expect to obtain from base...
Ch. 23.7 - Show the products you would expect to obtain by...Ch. 23.7 - Prob. 12PCh. 23.8 - What product would you expect from the following...Ch. 23.9 - What product would you expect From the following...Ch. 23.9 - Prob. 15PCh. 23.10 - Prob. 16PCh. 23.10 - Prob. 17PCh. 23.10 - Prob. 18PCh. 23.11 - Prob. 19PCh. 23.11 - Show how you might use an enamine reaction to...Ch. 23.12 - Prob. 21PCh. 23.12 - How would you prepare the following compound using...Ch. 23.SE - Prob. 23VCCh. 23.SE - Prob. 24VCCh. 23.SE - Prob. 25VCCh. 23.SE - The following molecule was formed by a Robinson...Ch. 23.SE - Prob. 27MPCh. 23.SE - Prob. 28MPCh. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Predict the product(s) and provide the mechanism...Ch. 23.SE - Knoevenagel condensation is a reaction involving...Ch. 23.SE - Azlactones are important starting materials used...Ch. 23.SE - Prob. 34MPCh. 23.SE - Isoleucine, another of the twenty amino acids...Ch. 23.SE - The first step in the citric acid cycle of food...Ch. 23.SE - Prob. 37MPCh. 23.SE - The Knoevenagel reaction is a carbonyl...Ch. 23.SE - The Darzens reaction invoIves a two-step,...Ch. 23.SE - The following reaction involves a hydrolysis...Ch. 23.SE - Prob. 41MPCh. 23.SE - Prob. 42MPCh. 23.SE - Prob. 43MPCh. 23.SE - Propose a mechanism for the following...Ch. 23.SE - Prob. 45MPCh. 23.SE - Prob. 46MPCh. 23.SE - Prob. 47MPCh. 23.SE - Prob. 48APCh. 23.SE - Prob. 49APCh. 23.SE - Prob. 50APCh. 23.SE - Prob. 51APCh. 23.SE - Prob. 52APCh. 23.SE - Prob. 53APCh. 23.SE - Prob. 54APCh. 23.SE - Prob. 55APCh. 23.SE - Prob. 56APCh. 23.SE - Prob. 57APCh. 23.SE - Prob. 58APCh. 23.SE - In the mixed Claisen reaction of cyclopentanone...Ch. 23.SE - Ethyl dimethylacetoacetate reacts instantly at...Ch. 23.SE - Prob. 61APCh. 23.SE - Prob. 62APCh. 23.SE - The so-called Wieland-Miescher ketone is a...Ch. 23.SE - Prob. 64APCh. 23.SE - Prob. 65APCh. 23.SE - Prob. 66APCh. 23.SE - What condensation products would you expect to...Ch. 23.SE - The following reactions are unlikely to provide...Ch. 23.SE - Fill in the missing reagents a-h in the following...Ch. 23.SE - Prob. 70APCh. 23.SE - Prob. 71APCh. 23.SE - Prob. 72APCh. 23.SE - Prob. 73AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tamoxifen is a drug used in the treatment of breast cancer. How would you prepare tamoxifen from benzene, the following ketone, and any other reagents needed?arrow_forwardH3C. 00 H3C. CH3 XII HO: OCH3 Aldehydes and ketones react reversibly with two equivalents of alcohol in the presence of an acid catalyst to give acetals. Alcohols are poor nucleophiles, and so protonation of the carbonyl oxygen is used to make the carbonyl carbon a stronger electrophile. Addition of the first equivalent of alcohol gives a hemiacetal, a hydroxyether. Addition of the second equivalent of alcohol is accompanied by loss of water to yield the product acetal. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions 2 CH₂OH HCI catalyst CH3 H3C H-OH₂ H3CO OCH3 CH3 H3C. H₂O: OCH3 CH3 H₂O Чаarrow_forward2) How would you synthesize the following compounds from cyclohexanone? CH₂Br CH₂C6H5 A Br B C D CH2CH2CO2Harrow_forward
- The compound shown was required for the preparation of fluorescent markers for biological compounds. How can it be synthesized from 2-chloro-3-hydroxy-4-methoxybenzaldehyde?arrow_forward19 Which of the following ketoesters can be formed by Dieckmann cyclization? D E B C بلا سلامarrow_forwardO-chem help!arrow_forward
- What ketones are prepared by the following reactions?arrow_forwardN,N-Diethyl-m-toluamide (DEET) is the active ingredient in many insect-repellent preparations. How might you synthesize this substance from m-bromotoluene?arrow_forward15-41 Compound A(C5Hh, is not optically active and cannot be separated into enantiomers. It reacts with bromine in carbon tetrachloride to discharge the purple color of bromine and form Compound B( C.FL Br,). When Compound A is treated with H., in the presence of a transition metal catalyst, it is converted to compound C(C5H10). When treated with HC1, compound A is converted to compound DtC.HyCl). Given this information, propose structural formulas for compounds A B, C, and D. Hint: There are at least three possibilities for Compound A and, in turn, three possibilities for Compounds B. C, and D.arrow_forward
- A step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forwardIdentify A, B, and C, intermediates in the synthesis of the five-membered ring called an α-methylene-γ-butyrolactone. This heterocyclic ring system is present in some antitumor agents.arrow_forwardKetoprofen, like ibuprofen, is an anti-inflammatory analgesic. How can ketoprofen be synthesized from the given starting material?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY