
Concept explainers
(a)
Interpretation:
Mechanism for the 1st step of the reaction has to be given along with the reason for the direction.
Concept Introduction:
Acid-base reaction:
The species that release proton or accept lone pair of electrons are called acids and the species that accept proton or donate lone pair of electrons are called base.
(b)
Interpretation:
The mechanism for the oxidation step has to be shown.
Concept introduction:
The palladium reagent used as electron transfer reagent to the phenolic oxygen.
(c)
Interpretation:
The mechanism of the respective step has to be given along with the stereochemistry.
Concept introduction:
Diels Alder reaction:
The Diels-Alder reaction is a
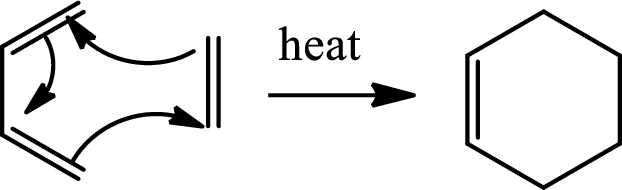
(d)
Interpretation:
Whether the product is racemic or single enantiomer that has to be determined.
Concept introduction:
Racemic mixture:
Racemic mixture is the mixture that has equal amounts of left and right handed enantiomers of the chiral molecule.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Over the past several decades, chemists have developed a number of synthetic methodologies for the synthesis of steroid hormones. One of these, developed by Lutz Tietze at the Institut für Organische Chemie der Georg-August-Universität, Göttingen, Germany, used a double Heck reaction to create ring B of the steroid nucleus. As shown in the following retrosynthetic analysis, a key intermediate in his synthesis is compound (1). Two Heck reaction disconnects of this intermediate give compounds (2) and (3). Compound (2) contains the aromatic ring that becomes ring A of estrone. Compound (3) contains the fused five- and six-membered rings that become rings C and D of estrone. Q.Show how your proposals for compounds (2) and (3) can be converted to compound (1). (Note: In the course of developing this synthesis, Tietze discovered that vinylic bromides and iodides are more reactive in Heck reactions than are aryl bromides and iodides.)arrow_forwardOver the past several decades, chemists have developed a number of synthetic methodologies for the synthesis of steroid hormones. One of these, developed by Lutz Tietze at the Institut für Organische Chemie der Georg-August-Universität, Göttingen, Germany, used a double Heck reaction to create ring B of the steroid nucleus. As shown in the following retrosynthetic analysis, a key intermediate in his synthesis is compound (1). Two Heck reaction disconnects of this intermediate give compounds (2) and (3). Compound (2) contains the aromatic ring that becomes ring A of estrone. Compound (3) contains the fused five- and six-membered rings that become rings C and D of estrone. Q. In the course of the double Heck reactions, two new chiral centers are created. Assume in compound (3), the precursor to rings C and D of estrone, that the fusion of rings C and D is trans and that the angular methyl group is above the plane of the ring. Given this stereochemistry, predict the stereochemistry of…arrow_forwardDraw the products and necessary reagents of the three step retrosynthetic reaction sequence shown below. Use wedge and dash bonds to indicate stereochemistry where appropriate. Ignore inorganic byproducts. Please choose from the following options for each of the reagents. (top reagents options) A. HNO3, cat. H2SO4 B. SO3, cat. H2SO4 C. CH3C(=O)Cl, AlCl3 D. Cl2, FeCl3 E. H2O, HCl (reagents option to the right) (top reagents options) A. HNO3, cat. H2SO4 B. SO3, cat. H2SO4 C. CH3C(=O)Cl, AlCl3 D. Cl2, FeCl3 E. H2O, HClarrow_forward
- Adjust the structures below to show the resulting intermediates.arrow_forwardSN1 reactions are better performed in protic solvents. Explain why the researchers could not perform the reaction using 100% water as solvent instead of 50% aqueous acetone.arrow_forwardMatch the drugs with the corresponding Phase 1 reaction that will happen appropriately with the highligthed portion of the drug. A: C4 of Benzene ring B: Methyl group of Nitrogen C: -NH-NH2 moiety D: N,N-dimethyl moiety E: Ring S CHOICES: A. Desulfuration B. Aromatic hydroxylation C. Hydrazo reduction D. Nitro reduction E. Sulfur oxidation F. Deamination G. N-demethylationarrow_forward
- Show an actual arrow-pushing mechanism for the transformation below, and briefly explain the observed regioselectivity.arrow_forward10.14 Complete the following reactions: Show the step-by-step process. Do not use shortcut methods. Make it as detailed as it can be. Encode (not hand-written)!arrow_forwardAlcohols react with sulfonyl chlorides to form sulfonate esters. Only the O-H bond of the alcohol is broken in the reaction, and so no inversion of configuration occurs. The resulting sulfonate esters are reactive in SN1 and SN2 reactions since the sulfonate group is a very weak base and is therefore a good leaving group.Draw curved arrows to show the movement of electrons in this step of the mechanism.arrow_forward
- Following is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. You have not studied the Baeyer-Villiger reaction (D to E). The mechanism involves nucleophilic reaction of the peroxyacid with the carbonyl followed by a rearrangement much like that involved in the hydroboration reaction ). Write a mechanism for this reaction.arrow_forwardFollowing is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. The tributyltin hydride, Bu3SnH, used in the conversion of (H) to (I) reacts via a radical chain reaction; the first step involves a reaction with a radical initiator to form Bu3Sn?. Suggest a mechanism for the rest of the reaction.arrow_forwardFollowing is an outline of the stereospecific synthesis of the “Corey lactone.” Professor E. J. Corey (Harvard University) describes it this way. “The first general synthetic route to all the known prostaglandins was developed by way of bicycloheptene intermediates. The design was guided by the requirements that the route be versatile enough to allow the synthesis of many analogs and also allow early resolution. This synthesis has been used on a large scale and in laboratories throughout the world; it has been applied to the production of countless prostaglandin analogs. Note: The wavy lines in compound C indicate that the stereochemistry of -Cl and -CN groups was not determined. Q. What is the function of sodium hydride, NaH, in the first step? What is the pK a of cyclopentadiene? How do you account for its remarkable acidity?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

