
(a)
Interpretation:
The types of
Concept introduction:
Functional groups:
The functional group is defined as an atom or group of atoms combined in a specific manner that gives the chemical properties of the organic compound and they are the main reason for the chemical reactivity of the compound. Compounds having similar functional groups undergoes similar type of reactions.
(b)
Interpretation:
The number of chiral centres present in estrone that has to be calculated.
Concept introduction:
Chiral centre:
Chiral centre is defined as an atom bonded to four different chemical species. It is a stereo centre that holds the atom in such way that the structure may not be superimposable to its mirror image. They give optical isomerism.
(c)
Interpretation:
Structural formula for the compounds
Concept introduction:
Structural formula:
The structural formula of a compound can be defined as a graphic representation of the molecular structure showing how the atoms are arranged and the
Retrosynthetic approach:
Retrosynthetic analysis is a technique for planning a synthesis mainly for complex organic molecules where the complex target molecule is reduced into a sequence of progressively simpler structures along a pathway which ultimately leads to the identification of a simple starting molecule or easily available from which the synthesis can be developed.
During retrosynthetic analysis the target molecule is symmetrically broken down by a combination of functional group interconversion and disconnection. This disconnection is related to breaking of carbon-carbon bond of a molecule to generate simpler fragments. The complete set of disconnections and functional group interconversions for a specified target molecule is what constitutes a retrosynthetic plan.
Heck reaction:
The Heck reaction is the
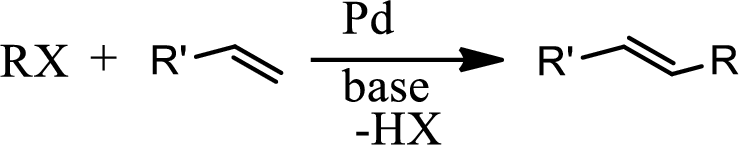
(d)
Interpretation:
The pathway of converting compounds
Concept introduction:
Heck reaction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
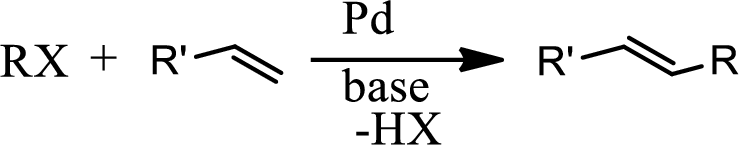
(e)
Interpretation:
The stereochemistry of compound
Concept introduction:
The Heck reaction is the chemical reaction of an unsaturated halide with an alkene in presence of a base and palladium catalyst to form a substituted alkene. The reaction is as follows,
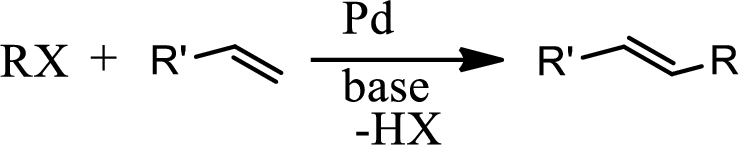
(f)
Interpretation:
The pathway of conversion of tertiary butyl ether to acetone to form estrone from compound
Concept introduction:
Oxidation of secondary alcohol:
Oxidation of alcohol to carbonyl is very difficult as the reaction is so fast that it always ends up by giving acid. Hence for secondary alcohol to get oxidised to carbonyl selectively PCC is used.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
Organic Chemistry
- Over the past several decades, chemists have developed a number of synthetic methodologies for the synthesis of steroid hormones. One of these, developed by Lutz Tietze at the Institut für Organische Chemie der Georg-August-Universität, Göttingen, Germany, used a double Heck reaction to create ring B of the steroid nucleus. As shown in the following retrosynthetic analysis, a key intermediate in his synthesis is compound (1). Two Heck reaction disconnects of this intermediate give compounds (2) and (3). Compound (2) contains the aromatic ring that becomes ring A of estrone. Compound (3) contains the fused five- and six-membered rings that become rings C and D of estrone. Q. In the course of the double Heck reactions, two new chiral centers are created. Assume in compound (3), the precursor to rings C and D of estrone, that the fusion of rings C and D is trans and that the angular methyl group is above the plane of the ring. Given this stereochemistry, predict the stereochemistry of…arrow_forwardExplain how benzaldehyde and dimedone reacts with each other, and then with the aminotriazole to form compound 1a in the presence of an acid catalyst. Provide a detailed reaction mechanism. During the development of the optimized procedure for the experiment, it was found out that compound 1b can also be produced from the same set of starting materials. Propose a detailed reaction mechanism for the formation of 1b. Explain your answer. What factor/s may drive the formation of 1b over 1a?arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
- Treatment of salicylaldehyde (2-hydroxybenzaldehyde) with bromine in glacial acetic acid at 0°C gives a compound with the molecular formula C7H4Br2O2, which is used as a topical fungicide and antibacterial agent. Propose a structural formula for this compoundarrow_forwardDescribe a synthetic plan with suitable justification of the sequence of reactions you would understate to complete synthesis of compound 1 from compounds 2,3,4. Suggest suitable reagentsarrow_forwardPyridine undergoes electrophilic aromatic substitution preferentially at the 3 position as illustrated by the synthesis of 3-nitropyridine. Under these acidic conditions, the species undergoing nitration is not pyridine, but its conjugate acid. Write resonance contributing structures for the intermediate formed by attack of NO2+ at the 2, 3, and 4 positions of the conjugate add of pyridine. From examination of these intermediates, offer an explanation for preferential nitration at the 3 position.arrow_forward
- Starting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.) m-Chlorobenzoic acidarrow_forwardPlease show the electron-flow mechanism of the general synthesis of Benzyl Chloride from Phenol. This involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shown.arrow_forwardDeduce a synthetic strategy for the synthesis of m-nitroacetanilide using retrosynthetic analysis. Explain the possible side product for the reaction and differentiate the final product and the side product by spectroscopic methodsarrow_forward
- Given this retrosynthetic analysis, propose a synthesis for labetalol from salicylic acid and benzyl chloride. [Note: The conversion of salicylic acid to (E) involves a Friedel-Crafts acylation in which the phenolic -OH must be protected by treatment with acetic anhydride to prevent the acylation of the -OH group. The protecting group is later removed by treatment with KOH followed by acidification.]arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)m-Nitrobenzenesulfonic acidarrow_forwardPlease show the electron-flow mechanism of the synthesis of benzyl chloride from phenol this involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shown.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

