
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 24, Problem 24.44P
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the product formed when CH3CH2C=CH is treated with each of the following sets of reagents: (a) H2O, H2SO4, HgSO4; and (b) R2BH, followed by H2O2, HO−.
(a) Draw the structure of the hemiacetal formed from one mole of benzaldehyde and one mole of
ethanol.
(b) Draw the structure of the acetal formed from one mole of benzaldehyde and two moles of ethanol.
(c) Draw the structure of 2-methoxy-2-butanol. What compounds could you prepare this from?
(d) Draw the structure of 3-methoxyl-2-butanol. What functional groups are present? Is this an acetal, a
hemiacetal, or neither? Explain.
(e) Identify the functional groups in the molecules shown below. Circle any acetals or hemiacetal, and
identify which they are.
0-
Draw the structure of the hydroxyaldehyde product from the self-aldol reaction of each of the following aldehydes: (a) propanal; (b) phenylethanal; (c) 3-phenylpropanal; (d) benzaldehyde.
Chapter 24 Solutions
ORGANIC CHEMISTRY
Ch. 24 - Prob. 24.1PCh. 24 - Prob. 24.2PCh. 24 - Problem 24.3
What unsaturated carbonyl compound is...Ch. 24 - Prob. 24.4PCh. 24 - Prob. 24.5PCh. 24 - Prob. 24.6PCh. 24 - Problem 24.7
Draw the products formed in each...Ch. 24 - Problem 24.8
Draw the products formed in the...Ch. 24 - Prob. 24.9PCh. 24 - Prob. 24.10P
Ch. 24 - Prob. 24.11PCh. 24 - Prob. 24.12PCh. 24 - Prob. 24.13PCh. 24 - Prob. 24.14PCh. 24 - Prob. 24.15PCh. 24 - Problem 24.16
What ester is formed when each...Ch. 24 - Prob. 24.17PCh. 24 - Prob. 24.18PCh. 24 -
Draw the products of each reaction.
a. b.
Ch. 24 - Problem 24.20
Two steps in a synthesis of the...Ch. 24 - Prob. 24.21PCh. 24 - Problem 24.22
Which of the following compounds can...Ch. 24 - Prob. 24.23PCh. 24 - Problem 24.24
What starting materials are needed...Ch. 24 - Problem 24.25
Draw the products when each pair of...Ch. 24 - Prob. 24.26PCh. 24 - Problem 24.27
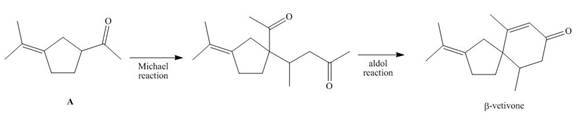
What starting materials are needed...Ch. 24 - Prob. 24.28PCh. 24 - 24.29 What steps are needed to convert A to B?
Ch. 24 - Prob. 24.30PCh. 24 - 24.31 Draw the product formed in each directed...Ch. 24 - Prob. 24.32PCh. 24 - 24.33 What starting materials are needed to...Ch. 24 - Prob. 24.34PCh. 24 - Prob. 24.35PCh. 24 - 24.36 Identify the structures of C and D in the...Ch. 24 - Prob. 24.37PCh. 24 - Prob. 24.38PCh. 24 - 24.39 Draw the product formed from a Claisen...Ch. 24 - Prob. 24.40PCh. 24 - 24.41 Even though B contains three ester groups, a...Ch. 24 - Prob. 24.42PCh. 24 - Prob. 24.43PCh. 24 - 24.44 Vetivone is isolated from vetiver, a...Ch. 24 - Draw the product of each Robinson annulation from...Ch. 24 - Prob. 24.46PCh. 24 - 24.47 Draw the organic products formed in each...Ch. 24 - 24.48 Fill in the lettered reagents needed for...Ch. 24 - Prob. 24.49PCh. 24 - Prob. 24.50PCh. 24 - Prob. 24.51PCh. 24 - 24.52 Draw a stepwise mechanism for the following...Ch. 24 - Prob. 24.53PCh. 24 - Prob. 24.54PCh. 24 - Prob. 24.55PCh. 24 - Prob. 24.56PCh. 24 - Prob. 24.57PCh. 24 - Prob. 24.58PCh. 24 - Prob. 24.59PCh. 24 - 24.60 Devise a synthesis of each compound from the...Ch. 24 - 24.61 Devise a synthesis of each compound from...Ch. 24 - 24.62 Devise a synthesis of each compound from ,...Ch. 24 - Prob. 24.63PCh. 24 - Prob. 24.64PCh. 24 - 24.65 Answer the following questions about...Ch. 24 - Prob. 24.66PCh. 24 - Prob. 24.67PCh. 24 - Prob. 24.68PCh. 24 - 24.69 Devise a stepwise mechanism for the...Ch. 24 - 24.70 Draw a stepwise mechanism for the following...Ch. 24 - Prob. 24.71PCh. 24 - Prob. 24.72PCh. 24 - Prob. 24.73P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Name the structure. Heating compound A in the presence of H2SO4 results in which product?arrow_forward22. Provide the following: 1) the major organic product of each reaction shown, and ii) the specific name of the reaction in the first step. aniline AICI (if o,p-substituted products are possible, only provide the major para-substituted product, otherwise, only provide the meta-substituted product) 12, HNO (if o,p-substituted products are possible, only provide the major para-substituted product, otherwise, only provide the meta-substituted product) end a chatarrow_forwardDraw the products formed when A and B are treated with each of the following reagents: (a) Br2, FeBr3; (b) HNO3, H2SO4; (c) CH3CH2COCl, AlCl3.arrow_forward
- A synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardWhat steps are needed to convert benzene into p-isobutylacetophenone, a synthetic intermediate used in the synthesis of the anti-infl ammatory agent ibuprofen.arrow_forwardWhat product is formed when each compound is treated with either Ag2O, NH4OH or Na2Cr2O7, H2SO4, H2O?arrow_forward
- β-Vetivone is isolated from vetiver, a perennial grass that yields a variety ofcompounds used in traditional eastern medicine, pest control, and fragrance. In one synthesis, ketone A is converted to β-vetivone by a two-step process: Michael reaction, followed by intramolecular aldol reaction. (a) What Michael acceptor is needed for the conjugate addition? (b) Draw a stepwise mechanism for the aldol reaction, which forms the six-membered ring.arrow_forwardDraw the products formed when each compound is treated with HNO3 and H2SO4.State whether the reaction occurs faster or slower than a similar reaction with benzene.arrow_forward(a) Draw the structure of the hemiacetal formed from one mole of benzaldehyde and one mole of ethanol. (b) Draw the structure of the acetal formed from one mole of benzaldehyde and two moles of ethanol. (c) Draw the structure of 2-methoxy-2-butanol. What compounds could you prepare this from?arrow_forward
- Draw the products formed when p-methylaniline (p-CH3C6H4NH2) istreated with following reagent. CH3COCl, AlCl3arrow_forwardOne step in the synthesis of occidentalol, a natural product isolated from the eastern white cedar tree, involved the following reaction. Identify the structure of A and show how A is converted to B.arrow_forwardIdentify the lettered compounds in each reaction sequence.Draw the product formed when phenylacetonitrile (C6H5CH2CN) istreated with below reagent. H2O, −OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY