
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.4, Problem 2.7P
Predict which of the following
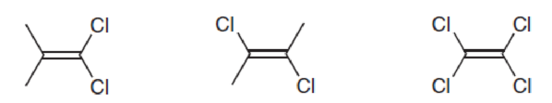
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following compounds will show
intense signals in 2250CM^-1 and why?
OH
H3C-
H3C-
EN
-CH3
H3C
H3C
Which of the following bonds would produce the strongest absorption?
C=N
C≡C
C=O
sp2C—H
C—O
4- A compound with the general formula C4H6 and having two types of signals in the HNMR spectrum is
2-Butyne.
Q/ true or false
5- The compound C4H9Br that has only one signal in the NMR spectrum is the compound
tert-butyl bromide
6- The proton of the aldehyde group appears at a low field because the movement of electrons in the
C=O bond is generated An inscribed magnetic field opposite to the external applied magnetic field.
Chapter 2 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
A compound that contains only C and H was burned in excess O2 to give CO2 and H2O. When 0.270 g of the compound...
General Chemistry: Atoms First
48. Are these data sets on chemical changes consistent with the law of conservation of mass?
a. A 12.8-g sample...
Introductory Chemistry (5th Edition) (Standalone Book)
A flat plate is of planar dimension 1m0.75m. For parallel laminar flow over the plate, calculate the ratio of t...
Fundamentals of Heat and Mass Transfer
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
What is the wavelength, in nanometers, of light with an energy content of 2112 kJ/mol? In what portion of the e...
General Chemistry: Principles and Modern Applications (11th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following compounds absorbs at the Longest Wavelength (Highest λmax)?arrow_forwardmolecular formula: C5H9N 1. Given the molecular formula, label important peaks on the spectrum and explain how you determined the Structural Formula for your assigned compound.arrow_forwardCompound 1 has molecular formula C7H16. It shows three signals in the 1H-NMR spectrum, one at 0.85 ppm, one at 1.02 ppm, and one at 1.62 ppm. The relative integrals of these three signals are 6, 1, and 1, respectively. Compound 2 has molecular formula C7H14. It shows three signals in the 1H-NMR spectrum, one at 0.98 ppm, one at 1.36 ppm, and one at 1.55 ppm. The relative integrals of these three signals are 3, 2, and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forward
- Within what region of the electromagnetic spectrum do you expect this compound to absorb? CIRCLE one. < 400 nm 400 – 500 nm above 500 nm but lower than 700 nmarrow_forward1Compound 1 has molecular formula C7H16. It shows three signals in the 1H-NMR spectrum, one at 0.85 ppm, one at 1.02 ppm, and one at 1.62 ppm. The relative integrals of these three signals are 6, 1, and 1, respectively. Compound 2 has molecular formula C7H14. It shows three signals in the 1H-NMR spectrum, one at 0.98 ppm, one at 1.36 ppm, and one at 1.55 ppm. The relative integrals of these three signals are 3, 2, and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forwardThe C=C bond in 2-cyclohexenone (shown below) produces an unusually strong signal. Explain using resonance structures. 14.06a1 Which of the following explains why the C=Cond in 2-cyclohexenone produces an unusually strong signal. O Conjugation with the C=O results in resonance, giving the C=C bond some single bond character (making it weaker). Conjugation with the C=O results in resonance, making the C=C bond more polar than usual. O Conjugation with the C=O results in resonance, making the C=C bond less polar than usual. O Conjugation with the C=O results in resonance, giving the C=C bond some single bond character (making it stronger).arrow_forward
- For the following compound how many different signals would you see in the carbon NMR? (Assume that you can see them all.) 4 O 5 O 3 09 CO |arrow_forwardButyl ethanoate is partially responsible for the flavor and scent of apples. How many 1HNMR signals should it have? (digits only)arrow_forwardWhat is the reactant and the desired product? If you were looking at both of the IR spectrum, where would you confirm that the desired reaction had occured.arrow_forward
- Indicate the functional groups present in the IR spectra shown below (you may choose more than one answer): choices: N-H group C≡N group Alkene (C=C) group C=O group Aromatic ring group O-H group Alkyne (C≡C) grouparrow_forwardFor the following compound how many different signals would you see in the carbon NMR? (Assume that you can see them all.) 4 O 8 07arrow_forward5) Levothyroxine (containing 4 iodine atoms), an important prescribed medication, is a form of thyroid hormone thyroxine. It is a very important drug that is used to treat patients with thyroid hormone deficiency (hypothyroidism). List all the IR diagnostic peaks for Levothyroxine below by approximating the frequency of IR absorption (in cm ¹) for each functional group on the drug (list them below). Ignore the fingerprint region if it is not important НО. int NH₂ OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY