
(a)
Interpretation:
Propylene does not undergo free radical
Concept introduction:
In any free radical polymerization reaction generally, three steps are involved viz. initiation, propagation, and termination. In initiation step, free radicals are generated from either monomer or from another compound such as hydrogen peroxide or benzoyl peroxide called a radical initiator. In propagation, step radicals react with the monomer to grow the chain to build up ![]() ) is used to represents the movement of a single electron in a homolysis process.
) is used to represents the movement of a single electron in a homolysis process.
Answer to Problem 26.37P
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. The mechanism and products for these two competing steps are given below:
Explanation of Solution
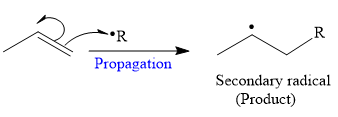
The mechanism for the propagation and its corresponding product for a given reaction is shown below:

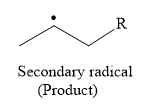
So in the propagation step, secondary radical is formed as a product.
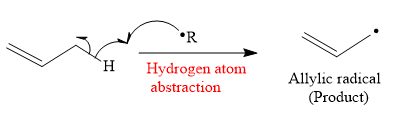
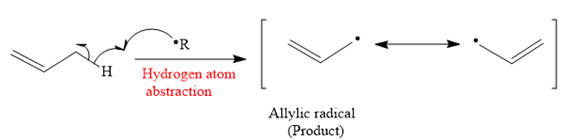
The mechanism for the hydrogen atom abstraction and its corresponding product in a given reaction is shown below:

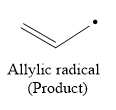
So in the hydrogen atom abstraction step, allylic radical is formed as a product.
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. The mechanism and products for these two competing steps are drawn.
(b)
Interpretation:
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. It is to be explained which step produces a more stable product.
Concept introduction:
In any free radical polymerization reaction generally, three steps are involved viz. initiation, propagation, and termination. In initiation step, free radicals are generated from either monomer or from another compound such as hydrogen peroxide or benzoyl peroxide called a radical initiator. In propagation, step radicals react with the monomer to grow the chain to build up polymer and this process is called a chain-growth polymerization or addition polymerization. Finally, in terminating step, the growth of polymer is stopped by two ways: combination or disproportionation. In combination step, two growing polymer radicals combine to form the uncharged product, polymer. The termination occurs when the one radical abstracts a hydrogen atom from another radical is called disproportionation. The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis. So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. The weakest bond possesses the smallest
) is used to represents the movement of a single electron in a homolysis process. The weakest bond possesses the smallest
Answer to Problem 26.37P
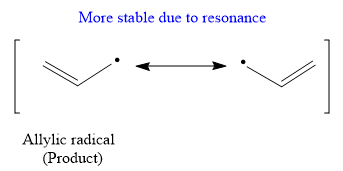
Due to resonance stabilization, an allylic radical is more stable than the secondary radical.
Explanation of Solution
The products of both these steps are:


The stability order of the radical is
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. It is explained which step produce a more stable product on the basis of the stability of radicals.
(c)
Interpretation:
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. It is to be explained why propylene is poor reactive in free radical polymerization.
Concept introduction:
In any free radical polymerization reaction generally, three steps are involved viz. initiation, propagation, and termination. In initiation step, free radicals are generated from either monomer or from another compound such as hydrogen peroxide or benzoyl peroxide called a radical initiator. In propagation, step radicals react with the monomer to grow the chain to build up polymer and this process is called a chain-growth polymerization or addition polymerization. In a such case for propagation step less stable radical must be formed. Finally, in terminating step, the growth of polymer is stopped by two ways: combination or disproportionation. In combination step, two growing polymer radicals combine to form the uncharged product, polymer. The termination occurs when the one radical abstracts a hydrogen atom from another radical is called disproportionation. The breaking of a covalent bond, whereby the electrons making up that bond are distributed equally to the atoms which are disconnected is known as the homolytic bond dissociation or homolysis. So in homolysis generally radicals are formed. In homolysis, a covalent bond is a breakdown equally and each atom acquires a single electron which is called a radical and a single barbed arrow (![]() ) is used to represents the movement of a single electron in a homolysis process. The weakest bond possesses the smallest bond dissociation energy. The stability order for radicals is
) is used to represents the movement of a single electron in a homolysis process. The weakest bond possesses the smallest bond dissociation energy. The stability order for radicals is
Answer to Problem 26.37P
In free radical polymerization reaction less stable radical must be formed to propagate the chain. But in given case more stable, allylic carbocation is formed which affects the propagation step results in rate of polymerisation is very low.
Explanation of Solution
The products of the both steps in free radical polymerization of propylene are given below:


Due to the formation of more stable, allylic radical in hydrogen atom abstraction step it will interfere with the formation of polymer.
In free radical polymerization reaction less stable radical must be formed to propagate the chain. But in given case more stable, allylic carbocation is formed which affects the propagation step results in rate of polymerisation is very low.
Propylene does not undergo free radical polymerization readily because there are two competing steps after initiation: propagation and hydrogen atom abstraction. It is explained why propylene is poor reactive in free radical polymerization.
Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





