
Concept explainers
(a)
Interpretation:
The E or Z configuration for the following molecule should be determined:
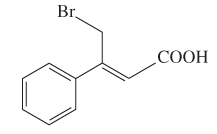
Concept introduction:
(b)
Interpretation:
The E or Z configuration for the following molecule should be determined:
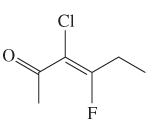
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
(c)
Interpretation:
The E or Z configuration for the following molecule should be determined:
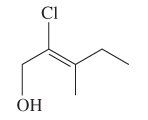
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be named as E and Z-configuration. The E-configuration stands for anti-configuration whereas Z stands for same side configuration. The determination of groups must be done on the basis of their molecular mass. The group or atom with high molecular mass must be numbered as 1 and other with 2. If both 1 numbered group/atom are placed at the same side they will consider as Z-configuration and in E-configuration these groups will be at anti-position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Selected Solutions Manual For General Chemistry: Principles And Modern Applications
- For each of the following molecules, assign whether the molecule is chiral or achiralarrow_forwardHow many chirality centers does the following molecule contain? Remember that C-H bonds are omitted from the skeletal structures of molecules.arrow_forwardPlace an asterisk (*) next to any chiral (stereogenic) carbon atoms in the following molecule:arrow_forward
- Classify the alcohol below as primary, secondary, or tertiary.arrow_forwardIndicate whether each of the following structures is an enantiomer of, a diastereomer of, or is identical to the structure above.arrow_forwardUsing wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has S stereochemical configuration.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
