
Concept explainers
(a)
Interpretation:
The hybridization of carbon atom, total C-C σ bonds and π bonds with 1â—¦,2â—¦,3â—¦ and 4â—¦ carbon atoms should be determined.
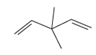
Concept introduction:
Hybridization is the process of mixing of atomic orbitals to form same energy and same shape hybrid orbitals which form covalent bond with overlapping of atomic orbitals of other atoms. The number of
1â—¦ orPrimary C atom = C atom which is bonded with one another C atom
2â—¦ orSecondary C atom= C atom which is bonded with two other C atoms
3â—¦ orTertiary C atom= C atom which is bonded with three other C atoms
4â—¦ or Quaternary C atom= C atom which is bonded with four other C atoms
(b)
Interpretation:
The hybridization of carbon atom, total C-C σ bonds and π bonds with 1â—¦, 2â—¦,3â—¦ and 4â—¦ carbon atoms should be determined.
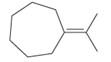
Concept introduction:
Hybridization is the process of mixing of atomic orbitals to form same energy and same shape hybrid orbitals which form covalent bond with overlapping of atomic orbitals of other atoms. The number of chemical bonds formed by the atom always determine type of hybridization. Sigma bonds could only form by hybrid orbitals whereas pi bonds are always formed by overlapping of un-hybrid orbitals hence hybridization is not part of pi bond formation.
1â—¦ or Primary C atom = C atom which is bonded with one another C atom
2â—¦ or Secondary C atom= C atom which is bonded with two other C atoms
3â—¦ or Tertiary C atom= C atom which is bonded with three other C atoms
4â—¦ or Quaternary C atom= C atom which is bonded with four other C atoms
(c)
Interpretation:
The hybridization of carbon atom, total C-C σ bonds and π bonds with 1â—¦, 2â—¦,3â—¦ and 4â—¦ carbon atoms should be determined.
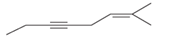
Concept introduction:
Hybridization is the process of mixing of atomic orbitals to form same energy and same shape hybrid orbitals which form covalent bond with overlapping of atomic orbitals of other atoms. The number of chemical bonds formed by the atom always determine type of hybridization. Sigma bonds could only form by hybrid orbitals whereas pi bonds are always formed by overlapping of un-hybrid orbitals hence hybridization is not part of pi bond formation.
1â—¦ or Primary C atom = C atom which is bonded with one another C atom
2â—¦ or Secondary C atom= C atom which is bonded with two other C atoms
3â—¦ or Tertiary C atom= C atom which is bonded with three other C atoms
4â—¦ or Quaternary C atom= C atom which is bonded with four other C atoms
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Selected Solutions Manual For General Chemistry: Principles And Modern Applications
- Identify the hybridization of carbon atoms numbered 1-6 in the structure below Carbons 1 and 3 are sp3, carbons 2 and 5 are sp2, carbons 4 and 6 are sp hybridized. Carbons 2 and 3 are sp3, carbons 4 and 5 are sp2, carbons 1 and 6 are sp hybridized. Carbons 1 and 2 are sp3, carbons 3 and 6 are sp2, carbons 4 and 5 are sp hybridized. Carbons 3 and 6 are sp3, carbons 4 and 5 are sp2, carbons 1 and 2 are sp hybridized. Carbons 4 and 5 are sp3, carbons 3 and 6 are sp2, carbons 1 and 2 are sp hybridized.arrow_forwardHow many chirality centers exist on this molecule? What is the absolute configuration of the primary alcohol in this structure? How many lone pairs are on the structure? How many sp2 hybridized atoms are on the structure?arrow_forwardThe structure of the molecule cyclohexene is Does the absorption of ultraviolet light by cyclohexene occur at longer or at shorter wavelengths than the absorption by benzene? Explain.arrow_forward
- Draw electron configuration diagrams for carbon in an unhybridized, sp3 -hybridized state, sp2 -hybridized state, and sp-hybridized state.arrow_forwardIdentify the number of sp3-hybridized carbon atoms in each of these isomers. 1. (CH3)2 CO 2. CH3CH2CHOarrow_forwardREDRAW PROPOXYPHENE and LABEL the following: a) circle and label ALL functional groups presence in the molecule b) PROPOXYPHENE is optically active. Mark on the structure ALL the chiral centre(s) using (*) c) State the electronegative atom(s) that is/are able to form hydrogen bonds with water molecules d) Show ALL LONE PAIR(S) using symbol (LP) in PROPOXYPHENE molecule e) Determine the type of hybridization and the geometrical shape for carbon Y and Z that shows in the picture given.arrow_forward
- Which of the following statements about an sp hybridized carbon is FALSE? a. It is divalent.b. It forms bonds that are linear.c. It has two p orbitals.d. It always forms triple bonds to carbon.arrow_forwardIs there much flexibility in the structure of benzene or can you assign a definite shape to the entire molecule? Compare the shape to that of cyclohexane.arrow_forwardWhich compound contains an sp2-hybridized carbon atom?arrow_forward
- Many reactions involve a change in hybridization of one or more atoms in the starting material. In following reaction, identify the atoms in the organic starting material that change hybridization and indicate the change.arrow_forwarda) what two types of bonds are present in alkanes? b) which of the two types of bonds is likely responsible for the absorption in the diagnostic region? c) what is the hybridization of the carbon atoms in all alkanes?arrow_forwardWhat is the absolute configuration of the C2 and C3 carbons in the following molecule?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning




