
Concept explainers
(a)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
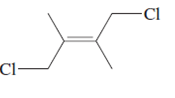
Concept introduction:
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the
(b)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
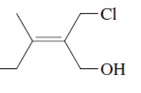
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(c)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
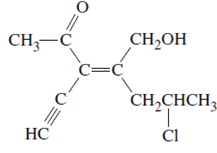
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
(d)
Interpretation:
The name of following molecule with appropriate stereochemical designation should be determined:
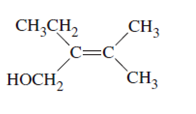
Concept introduction:
Alkenes are unsaturated hydrocarbons with double covalent bond between carbon-carbon atoms. On the basis of groups bonded with the double bonded carbon atoms, alkenes can be classified as E and Z-configuration.
The E-configuration stands for anti-configuration, whereas, Z-configuration stands for same side configuration.
The determination of configuration is done on the basis of the atomic/molecular mass of the atoms/groups attached to double bonded carbon atoms. If both higher atomic/molecular mass atom/groups are placed at the same side, then it is said to be Z-configuration and in E-configuration, these groups will be at anti-position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
General Chemistry: Principles and Modern Applications - With Solutions Manual and Modified MasteringChemistry Code
- Consider 1-bromo-2-methylpropane and draw the following. (a) The staggered conformation(s) of lowest energy (b) The staggered conformation(s) of highest energyarrow_forwardWhich conformation of cyclohexane has the greater steric strain?arrow_forwardDefine different cycloalkene nomenclature ?arrow_forward
- Which are the correct conformations of 2,2-dimethylpentane?arrow_forwardDraw the most stable conformation of the molecule while taking into account the approximate values of the energies of the following skew interactions:arrow_forwardWhat is the difference between a constitutional, confirgurational, and a conformational isomer?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
