
(a)
Interpretation:
The given set of triglycerides is fat or oil need to be identified.
Concept introduction:
Melting point of a compound is the temperature at which the compound starts to change from solid state to liquid state when temperature is raised. The total number of carbon atoms determines the melting point of the compound. Higher the carbon atoms and no unsaturation means higher is the melting point. If the melting point is low then the triglyceride will be in form of oil and if the melting point is high then the triglyceride will be fat. The generic structure of triglyceride can be drawn as,
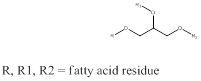
To identify: the triglyceride containing one palmitic acid and two stearic acid residues as fat or oil.
(b)
Interpretation:
The given set of triglycerides is fat or oil need to be identified.
Concept introduction:
Melting point of a compound is the temperature at which the compound starts to change from solid state to liquid state when temperature is raised. The total number of carbon atoms determines the melting point of the compound. Higher the carbon atoms and no unsaturation means higher is the melting point. If the melting point is low then the triglyceride will be in form of oil and if the melting point is high then the triglyceride will be fat. The generic structure of triglyceride can be drawn as,
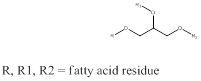
To identify: the triglyceride containing one oleic acid and two linoleic acid residues as fat or oil.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





