
(a)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes
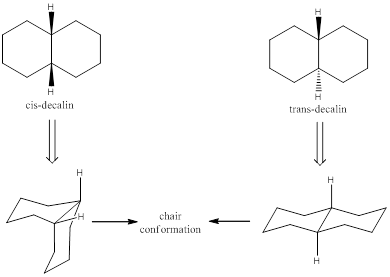
When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
(b)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes

When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
(c)
Interpretation:
Chair conformations and the position of substituents need to be identified for the given set of compounds.
Concept introduction:
Ring-flipping is a phenomenon known as ring inversion that involves rotation about the single bonds of cyclic conformers. Usually this happens in cyclohexane ring. When ring flip happens in the cyclohexane, the axially and equatorially substituted groups are inverted. The ring-flipping also happens in a fused ring system. Decalin is a fused ring system. There are two stereoisomers for decalin, namely cis-decalin and trans-decalin. If the substituent is facing away from the ring or which makes

When viewing the molecule from the top the bonds which are said to be in parallel with the viewing angle are in axial position and which are not parallel are known to be in equatorial position.
To draw and explain: chair conformation and identify the substituent position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 26 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





