
(a)
Interpretation:
Mechanism for the given transformation need to be drawn and if sodium hydroxide is present in large amount for transesterification.
Concept introduction:
Transesterification is a process in which the alcohol group present in the carboxylate of triglyceride is exchanged by another organic alcohol group. This can occur in presence of either acid or base as catalyst. The general scheme can be shown as,
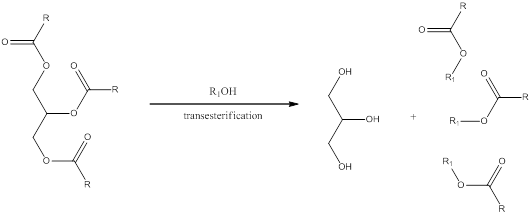
Please remember that transesterification using basic condition is done in few steps rather than acidic conditions.
In presence of excess base the transesterification does not happen instead hydrolysis occurs.
To draw: the mechanism for the given transformation.
(b)
Interpretation:
Mechanism for the given transformation need to be drawn and if sodium hydroxide is present in large amount for transesterification.
Concept Introduction:
Hydrolysis is a process in which the ester is converted to its respective carboxylic acid and alcohol in presence of aqueous acid or base. In case of triglyceride the hydrolysis results in formation of glycerol and three fatty acid units. In presence of other organic alcohol unit this hydrolysis occurs if the base is present in large amount rather than transesterification.
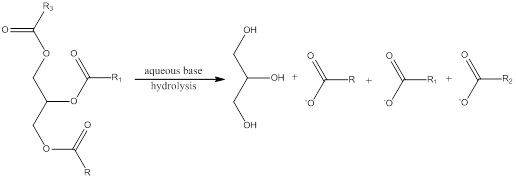
Trending nowThis is a popular solution!

Chapter 26 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





