
Concept explainers
(a)
Interpretation:
The principal product formed when
Concept introduction:
The general mechanism of electrophilic addition is as follows:
Step1:
Step2: Then the nucleophilic part of reagent adds to the carbocation to give the addition product.
These steps can be illustrated as follows:
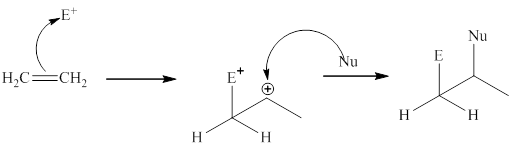
(b)
Interpretation:
The principal product formed in the monochlorination of propane should be predicted and written.
Concept introduction:
The reaction between chlorine and propane under ultraviolet light is the monohalogenation reaction. This is essentially a substitution reaction. Every possible monochlorinated products are formed. The major one is the one where chlorine is attached at the terminal carbon.
The principal product in the halogenation of
(c)
Interpretation:
The principal product when isopropyl alcohol is heated with benzoic acid should be predicted and written.
Concept introduction:
Heating alcohol and acid involve esterification reaction and an ester is formed along with the release of a water molecule.
The reaction can be illustrated as:

Where
The general mechanistic pathway for esterification as proposed by Fischer is as follows:
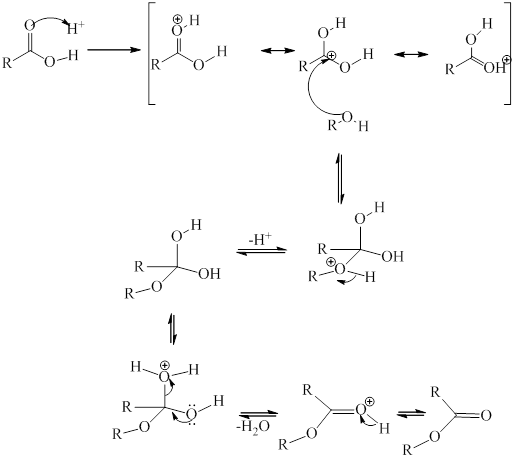
(d)
Interpretation:
The principal product when
Concept introduction:
Oxidation of alcohols can be done with an oxidizing reagent.
Tertiary alcohols cannot be oxidized as they do not have hydrogen; the secondary alcohols are oxidized to
While the primary alcohols oxidized to
The general formula of a tertiary, secondary and primary alcohol is represented as follows:
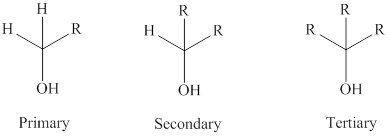
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Generl Chem Looself&mod Mst/et&stdy Crd Pkg, 11/e
- A hydrocarbon C5H12 gives only one mono-chlorination product. Identify the hydrocarbon.arrow_forward2,2,3,3-Tetrabromopentane can be prepared by an addition reaction of excess Br2 with an alkyne. Draw the structure of the alkyne and name it.arrow_forwardWrite the steps for formation of tetrachloromethane (CCl4) from the reaction of methane with Cl2 + hv.arrow_forward
- Suggest a net reaction for the production of nitroglycerin, C3H5(NO3)3, from glycerin, C3H5(OH)3.arrow_forwardThe sex attractant of the female tiger moth is an alkane of molecular formula C 18H 38. Is this molecule an acyclic alkane or a cycloalkane?arrow_forwardWhich of the following structural features could be found in a compound with formula C7H12?arrow_forward
- Give the structural formulae of all the chain isomers of C5H12.arrow_forwardWrite the polymerization reaction in which acetylene (C2H2) produces polyacetylene. Show the structural formulae of the monomers and the polymer.arrow_forwardThere are eight structures with the formula C3H7NO in whichthe O is part of a carbonyl group. Draw the structures andidentify the functional groups in each.arrow_forward
- 7. Give two structural formulas each for poly- and heterofunctional compounds of the composition C4H6O4.arrow_forwardGlucose, C6H12O6, contains an aldehyde group but exist predominantly in the form of the cyclic hemiacetal show below. A cyclic hemiacetal is formed when the —OH group of one carbon bonds to the carbonyl group of another carbon. Identify which carbon provides the —OH group and which provides the —CHO? Give a functional isomer of glucose and draw its structure.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning



