
(a)
Interpretation:
The structure of the product for the given reaction should be determined.
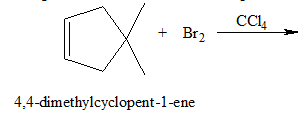
Concept introduction:
The halogenation of
In the halogenation reaction, inert solvents such as
A nucleophile is the Lewis base that donates a pair of an electron to form a covalent bond with the electrophile. An electrophile is the Lewis acid that accepts the electron to form a bond with the nucleophile.
(b)
Interpretation:
The structure of the product for the given reaction should be determined.
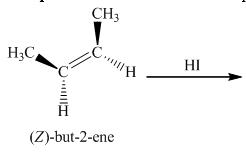
Concept introduction:
Reaction of alkenes with hydrogen halide is an example of an addition reaction. In this reaction, halogen and hydrogen atom adds across the carbon-carbon double bond to give a saturated compound.
(c)
Interpretation:
The structures of the product for the given reaction should be determined.
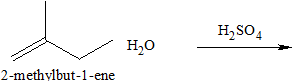
Concept introduction:
The addition on the alkene is the electrophilic addition reaction. In this reaction, electrophile reacts with carbon-carbon double of the alkene which results in the formation of
The addition of water to the alkene in the presence of strong acid which results in the formation of alcohol. This is the type of hydration of alkene.
A nucleophile is the Lewis base that donates a pair of an electron to form a covalent bond with the electrophile. An electrophile is the Lewis acid that accepts the electron to form a bond with the nucleophile.
Want to see the full answer?
Check out a sample textbook solution
Chapter 27 Solutions
Generl Chem Looself&mod Mst/et&stdy Crd Pkg, 11/e
- Write the structure of the major products in the following reactions and name them.arrow_forwardQuestion:In the process of organic synthesis, explain the concept of stereoselectivity and how it is influenced by factors such as reaction conditions, substrate structure, and the choice of catalyst. Provide specific examples to support your explanation.arrow_forwardComplete the following transformations by indicating the reactant structure, all necessary reagents, or the major organic product(s).arrow_forward
- The proper reaction scheme by indicating the reactant structure, all necessary reagents, or the major organic product(s) for each indicated transformation.arrow_forwardChemistry what is the major organic product of each of the following reactions.arrow_forwardPredict the products of reaction, if any, of isopropyl alcohol with each of the following reagents:arrow_forward
- Following are the steps in the industrial synthesis of glycerin. Provide structures for all intermediate compounds (AD) and describe the type of mechanism by which each is formed.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forwardEach of the following reactions has been described in the chemical literature and involves an organic reactant somewhat more complex than those we have encountered so far. Nevertheless, on the basis of the topics covered in this chapter, you should be able to write the structure of the principal organic product of each reaction.arrow_forward
- Give the structure of the alkenes which will generate the following products under the reaction conditions indicatedarrow_forwardGive a specific example of halogenation of alkenes including the starting material (substrate) and the major and minor products. Please state the IUPAC names of the products.arrow_forwardGive the major organic product generated by each of the following reactionsarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co


