
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27.SE, Problem 50AP
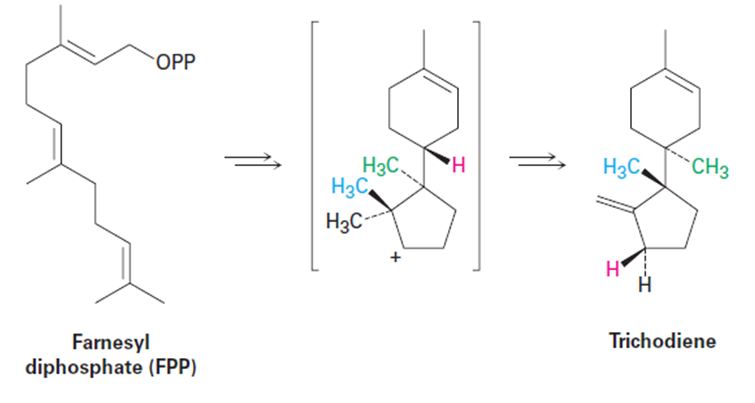
Propose a mechanism for the biosynthesis of the sesquiterpenoid trichodiene from farnesyl diphosphate. The process involves cyclization to give an intermediate secondary carbocation, followed by several carbocation rearrangements.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Treatment of trans-2-chlorocyclohexanol with NaOH yields 1, 2-epoxy-cyclohexane, but reaction of the cis isomer under the same conditions yields cyclohexanone. Propose mechanisms for both reactions, and explain why the different results are obtained.
Propose a mechanism for the acid-catalyzed hydration of methylidenecyclohexane to give 1-methylcyclohexanol. Which step in your mechanism is rate-determining?
Chapter 27 Solutions
Organic Chemistry
Ch. 27.1 - Carnauba wax, used in floor and furniture...Ch. 27.1 - Draw structures of glyceryl tripalmitate and...Ch. 27.2 - Prob. 3PCh. 27.2 - Write the saponication reaction of glyceryl...Ch. 27.4 - Prob. 5PCh. 27.5 - Prob. 6PCh. 27.5 - Prob. 7PCh. 27.6 - Draw the following molecules in chair...Ch. 27.6 - Lithocholic acid is an A–B cis steroid found in...Ch. 27.7 - Prob. 10P
Ch. 27.SE - Prob. 11VCCh. 27.SE - Propose a biosynthetic pathway for the...Ch. 27.SE - Identify the following fatty acid, and tell...Ch. 27.SE - Prob. 14MPCh. 27.SE - Prob. 15MPCh. 27.SE - Prob. 16MPCh. 27.SE - Prob. 17APCh. 27.SE - Fats can be either optically active or optically...Ch. 27.SE - Prob. 19APCh. 27.SE - Show the products you would expect to obtain from...Ch. 27.SE - Draw a Newman projection along the C2-C3 bond of...Ch. 27.SE - The plasmalogens are a group of lipids found in...Ch. 27.SE - Prob. 23APCh. 27.SE - Prob. 24APCh. 27.SE - Prob. 25APCh. 27.SE - Prob. 26APCh. 27.SE - Prob. 27APCh. 27.SE - Prob. 28APCh. 27.SE - Prob. 29APCh. 27.SE - Assume that acetyl CoA containing a 14C isotopic...Ch. 27.SE - Assume that acetyl CoA containing a 14C isotopic...Ch. 27.SE - Assume that acetyl CoA containing a 14C isotopic...Ch. 27.SE - Assume that acetyl CoA containing a 14C isotopic...Ch. 27.SE - Prob. 34APCh. 27.SE - Draw the most stable chair conformation of...Ch. 27.SE - Draw the most stable chair conformation of...Ch. 27.SE - Prob. 37APCh. 27.SE - Prob. 38APCh. 27.SE - Prob. 39APCh. 27.SE - What product would you obtain by reduction of...Ch. 27.SE - Prob. 41APCh. 27.SE - Eleostearic acid, C18H30O2, is a rare fatty acid...Ch. 27.SE - Prob. 43APCh. 27.SE - Prob. 44APCh. 27.SE - Propose a synthesis of diethylstilbestrol (Problem...Ch. 27.SE - Prob. 46APCh. 27.SE - Cembrene, C20H32, is a diterpenoid hydrocarbon...Ch. 27.SE - α-Fenchone is a pleasant-smelling terpenoid...Ch. 27.SE - Prob. 49APCh. 27.SE - Propose a mechanism for the biosynthesis of the...
Additional Science Textbook Solutions
Find more solutions based on key concepts
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
Fully developed conditions are known to exist for water flowing through a 25-nim-diameer tube at 0.01 kg/s and ...
Fundamentals of Heat and Mass Transfer
Write a Lewis formula for each of the following organic molecules: C2H3Cl (vinyl chloride: starting material fo...
Organic Chemistry - Standalone book
What is the pH range for acidic solutions? For basic solutions?
Introduction to Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Benzyl bromide is converted into benzaldehyde by heating in dimethyl sulfoxide. Propose a structure for the intermediate, and show the mechanisms of the two steps in the reaction.arrow_forwardThe pyrolysis of acetic esters to give an alkene and acetic acid is thought to involve a planar transition state and cyclic redistribution of (4n + 2) electrons. Propose a mechanism for pyrolysis of the following ester.arrow_forwardFollowing is a retrosynthetic analysis for an intermediate in the industrial synthesis of vitamin A. (a) Addition of one mole of HCl to isoprene gives 4-chloro-2-methyl-2-butene as the major product. Propose a mechanism for this addition and account for its regioselectivity. (b) Propose a synthesis of the vitamin A precursor from this allylic chloride and ethyl acetoacetate.arrow_forward
- Ethylene oxide is the starting material for the synthesis of 1,4-dioxane. Propose a mechanism for each step in this synthesis.arrow_forwardNonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardKnoevenagel condensation is a reaction involving an active methylene compound (a CH2 flanked by two electron-withdrawing groups) and an aldehyde and ketone. Propose a mechanism for the reaction below.arrow_forward
- When cis-4-chlorocyclohexanol is treated with sodium hydroxide in ethanol, it gives mainly the substitution product trans-1,4-cyclohexanediol (1). Under the same reaction conditions, trans-4-chlorocyclohexanol gives 3-cyclohexenol (2) and the bicyclic ether (3). (a) Propose a mechanism for formation of product (1), and account for its configuration. (b) Propose a mechanism for formation of product (2). (c) Account for the fact that the bicyclic ether (3) is formed from the trans isomer but not from the cis isomer.arrow_forwardTreatment of p-tert-butylphenol with a strong acid such as H2SO4 yields phenol and 2-methylpropene. Propose a mechanism.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License