
Concept explainers
Show the products you would expect to obtain from reaction of glyceryl Trioleate with the following reagents:
(a) Excess Br2 in CH2Cl2
(b) H2/Pd
(c) NaOH/H2O
(d) O3, then Zn/CH3CO2H
(e) LiAlH4, then H3O1
(f) CH3MgBr, then H3O1
a) Excess Br2 in CH2Cl2
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with bromine in excess, addition of bromine to all the olefinic double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is
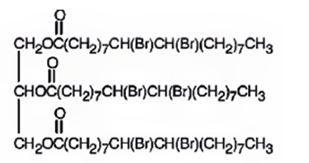
Explanation of Solution
Glyceryl trioleate has three olefinic diuble bonds. When glyceryl trioleate is treated with excess bromine in CH2Cl2 addition of bromine takes place to all the three double bonds in the molecule.
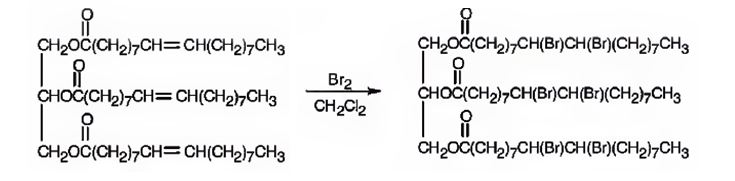
The product expected to be formed when glyceryl trioleate reacts with excess bromine in CH2Cl2 is

b) H2/Pd
Interpretation:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is to be given.
Concept introduction:
When compounds containing more than one olefinic double bond are treated with H2/Pd, reduction occurs and addition of hydrogen to all the double bonds in the molecule takes place.
To give:
The product expected to be formed when glyceryl trioleate reacts with H2/Pd.
Answer to Problem 20AP
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is
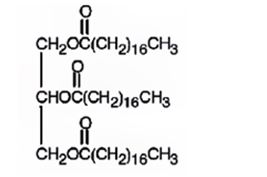
Explanation of Solution
When glyceryl trioleate is treated with H2/Pd, addition of hydrogen takes place to the three double bonds in the molecule to yield a saturated compound.
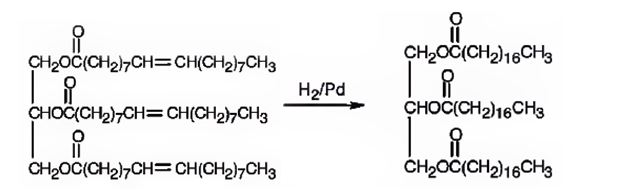
The product expected to be formed when glyceryl trioleate reacts with H2/Pd is

c) NaOH/H2O
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are to be given.
Concept introduction:
When an oil or fat is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium salt of the acid.
To give:
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.
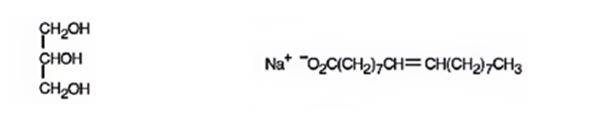
Explanation of Solution
When glyceryl trioleate is treated with aqueous NaOH, the ester linkages in them get hydrolyzed to yield glycerol and sodium oleate.
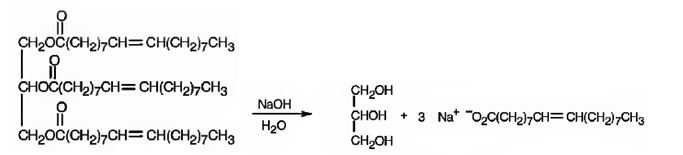
The products expected to be formed when glyceryl trioleate reacts with NaOH/H2O are given below.

d) O3, then Zn/CH3CO2H
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are to be given.
Concept introduction:
When a compound with double bond is treated with ozone and then with Zn/ CH3COOH, the double bond gets cleaved and the products have an oxygen atom attached to each carbon originally in the double bond.
To give:
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

Explanation of Solution
When glyceryl trioleate is treated with O3 and then with Zn/CH3COOH, ozone adds to the three double bonds to yield a triozonide which is cleaved when treated with Zn/CH3COOH to give two different aldehydes as products. The aldehyde carbons are present in a double bond in glyceryl trioleate.
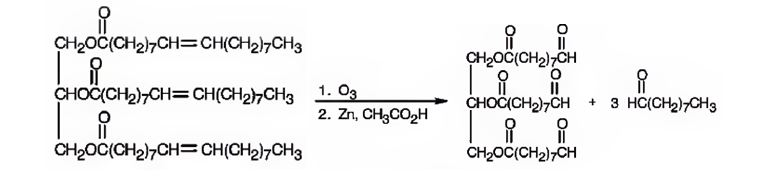
The products expected to be formed when glyceryl trioleate reacts with O3, then with Zn/CH3COOH are given below.

e) LiAlH4, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are to be given.
Concept introduction:
When oils and fats are reduced with LiAlH4, then with H3O+, the eater linkages are reduced to give glycerol and the free acid. Double bonds are not reduced by LiAlH4.
To give:
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.
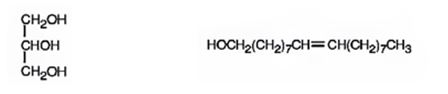
Explanation of Solution
When glyceryl trioleate is treated with LiAlH4 and then with H3O+ the ester linkages in it are reduced to alcohols. LiAlH4 does not reduce the double bonds in glyceryl trioleate.
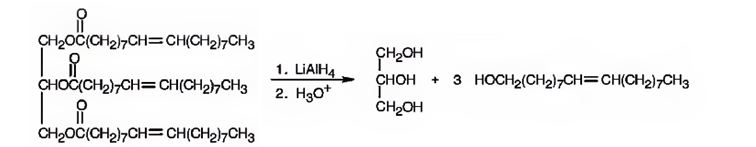
The products expected to be formed when glyceryl trioleate reacts with LiAlH4, then with H3O+ are given below.

f) CH3MgBr, then H3O+
Interpretation:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are to be given.
Concept introduction:
Esters when treated with a Grignard reagent and then with H3O+, react with two equivalents of the Grignard reagent to yield a tertiary alcohol. In oils and fats addition of Grignard reagent will take place to all the three carbonyl groups in the ester.
To give:
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+.
Answer to Problem 20AP
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.
Explanation of Solution
When glyceryl trioleate is treated with CH3MgBr, addition to carbonyl carbons takes place. The addition product when hydrolyzed with H3O+ yields three equivalents of a tertiary alcohol and glycerol as products.
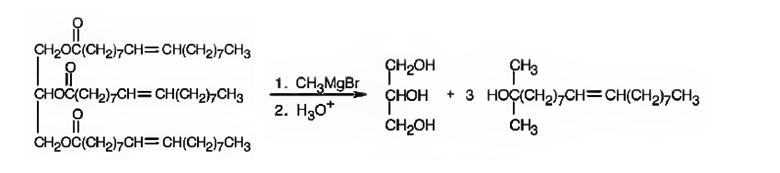
The products expected to be formed when glyceryl trioleate reacts with CH3MgBr, then with H3O+ are given below.

Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry - 4th edition
Organic Chemistry (9th Edition)
General, Organic, & Biological Chemistry
Chemistry: Atoms First
Living By Chemistry: First Edition Textbook
- Naturally occurring compounds called cyanogenic glycosides, such as lotaustralin, release hydrogen cyanide, HCN, when treated with aqueous acid. The reaction occurs by hydrolysis of the acetal linkage to form a cyanohydrin, which then expels HCN and gives a carbonyl compound. (a) Show the mechanism of the acetal hydrolysis and the structure of the cyanohydrin that results. (b) Propose a mechanism for the loss of HCN, and show the structure of the carbonyl compound that forms.arrow_forward(R)-Pulegone is converted to (R)-citronellic acid by addition of HCl followed by treatment with NaOH. Propose a mechanism for each step in this transformation and account for the regioselectivity of HCl addition.arrow_forwardHeating toluene in the presence of KMnO4 followed by acification with HCl, converts toluene into benzoic acid. Using this information and any other reactions discussed in this course, show a sequence of reactions showing howing toluene can be converted to p-aminobenzoic acid.arrow_forward
- 6 Please provide the correct answers. Thanks in advancearrow_forwardGive the product of the following reactions: (a) α-d-allopyranose + excess acetic anhydride and NaOAcarrow_forwardThe following scheme shows the retrosynthetic analysis of 1-isopentylaziridine to form series of intermediates via Functional Group Inversion (FGI). Choose the correct reagents of N-S for the respective conversionarrow_forward
- Iodine monochloride, ICl, a black crystalline solid with an mp of 27.2°C and a bp of 97°C, is prepared by mixing equimolar amounts of I2 and Cl2. Propose a mechanism for the iodination of 3-aminobenzoic acid by this reagent.arrow_forwardFollowing is a retrosynthesis for the coronary vasodilator ganglefene. (a) Propose a synthesis for ganglefene from 4-hydroxybenzoic acid and 3-methyl-3-buten-2-one. (b) Is ganglefene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardThe formation of Br2 from NBS first involves the reaction of NBS with HBr to form an iminol intermediate and molecular bromine. The intermediate then undergoes acid-catalyzed tautomerism to form succinimide, the byproduct of the reaction. Propose a curved-arrow mechanism for the conversion of NBS into succinimide that also accounts for the formation of Br2.arrow_forward
- Naturally occurring compounds called terpenoids, which we'll discuss in Section 27-5, are biosynthesized by a pathway that involves loss of CO2 from 3-phosphomevalonate 5-diphosphate to yield isopentenyl diphosphate. Use curved arrows to show the mechanism of this reaction.arrow_forwardThe following transformation involves a series of four consecutive Heck reactions and the formation of the four-ring steroid nucleus (Section 26.4) as a racemic mixture. Propose structural formulas for the palladium-containing intermediates involved in this reaction.arrow_forwardTreatment of pentanedioic (glutaric) anhydride with ammonia at elevated temperature leads to a compound of molecular formula C5H7NO2. What is the structure of this product? [Hint: You need to think about the reactivity not only of acid anhydrides but also of amides and carboxylic acids]arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

