
Concept explainers
Use Figure 3.4 to specify the region of the
Hint: Change each wavelength to meters before making the comparison.
- a. 2.0 cm
- b. 50 um
- c. 400 nm
- d. 150 mm
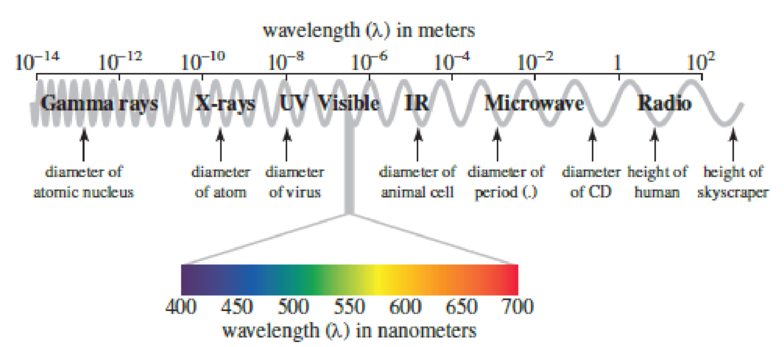
Figure 3.4
The electromagnetic spectrum, highlighting the visible region. Note that 10x is equivalent to 1 × 10x.
(a)
Interpretation:
The region of the electromagnetic spectrum for the given wavelength has to be specified.
Concept-Introduction:
Wavelength: The distance between successive peaks in a wave and measured in units of length.
Frequency: The number of waves passed a point in a certain amount of time.
Relation between wavelength and frequency:
Where, c is constant, the value of c is
Conversion of centimeter into meter (cm to m):
Explanation of Solution
The wavelength is
Conversion of centimeter to meter is,
Therefore, the given value of wavelength in meter is
(b)
Interpretation:
The region of the electromagnetic spectrum for the given wavelength has to be specified.
Concept-Introduction:
Wavelength: The distance between successive peaks in a wave and measured in units of length.
Frequency: The number of waves passed a point in a certain amount of time.
Relation between wavelength and frequency:
Where, c is constant, the value of c is
Conversion of micrometer into meter (
Explanation of Solution
The wavelength is
Conversion of micrometer to meter is,
Therefore, the given value of wavelength in meter is
(c)
Interpretation:
The region of the electromagnetic spectrum for the given wavelength has to be specified.
Concept-Introduction:
Wavelength: The distance between successive peaks in a wave and measured in units of length.
Frequency: The number of waves passed a point in a certain amount of time.
Relation between wavelength and frequency:
Where, c is constant, the value of c is
Conversion of nanometer into meter (nm to m):
Explanation of Solution
The given wavelength is
Conversion of nanometer to meter is,
Therefore, the given value of wavelength in meter is
(d)
Interpretation:
The region of the electromagnetic spectrum for the given wavelength has to be specified.
Concept-Introduction:
Wavelength: The distance between successive peaks in a wave and measured in units of length.
Frequency: The number of waves passed a point in a certain amount of time.
Relation between wavelength and frequency:
Where, c is constant, the value of c is
Conversion of millimeter into nanometer (mm to m):
Explanation of Solution
The given wavelength is
Conversion of millimeter to meter is,
Therefore, the given value of wavelength in meter is
Want to see more full solutions like this?
Chapter 3 Solutions
CHEMISTRY IN CONTEXT
Additional Science Textbook Solutions
Chemistry: Atoms First
EBK INTRODUCTION TO CHEMISTRY
Organic Chemistry (9th Edition)
Organic Chemistry
- One type of solar radiation in the upper atmosphere has a frequency of 7.898 1014 Hz; another type has a frequency of 1.20 1015 Hz. (a) In what region of the electromagnetic spectrum does this solar radiation occur? (b) Which of the two types of radiation has the shorter wavelength? Explain your answer.arrow_forwardThe spectra of hydrogen and of calcium are shown in Figure 6.13. What causes the lines in these spectra? Why are the colors of the lines different? Suggest a reason for the observation that the spectrum of calcium is more complicated than the spectrum of hydrogen.arrow_forwardight waves move through space at a speed of ters per second.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning





