
(a)
Interpretation:
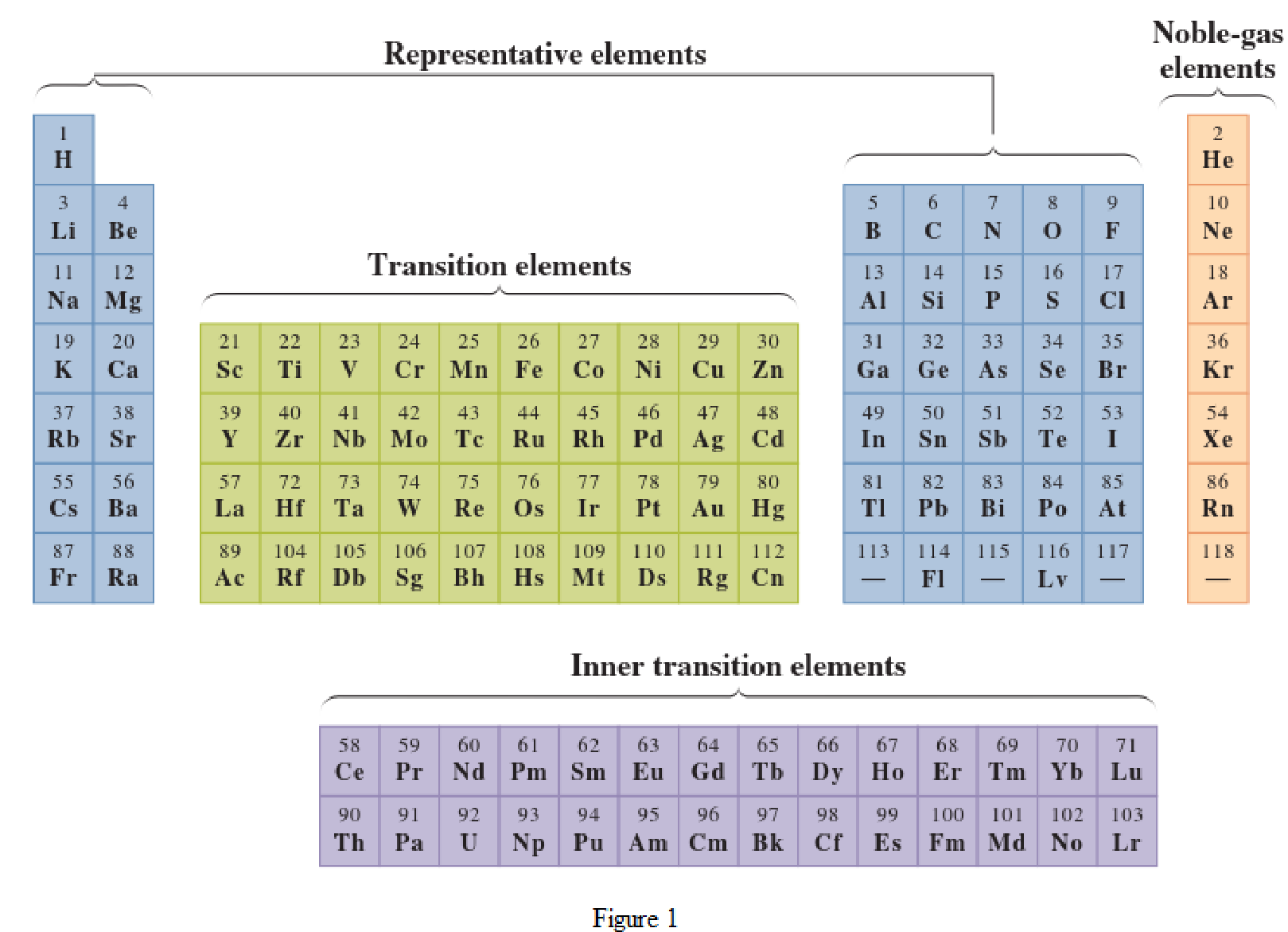
In the given periodic table, how many elements those are highlighted which represent inner
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(b)
Interpretation:
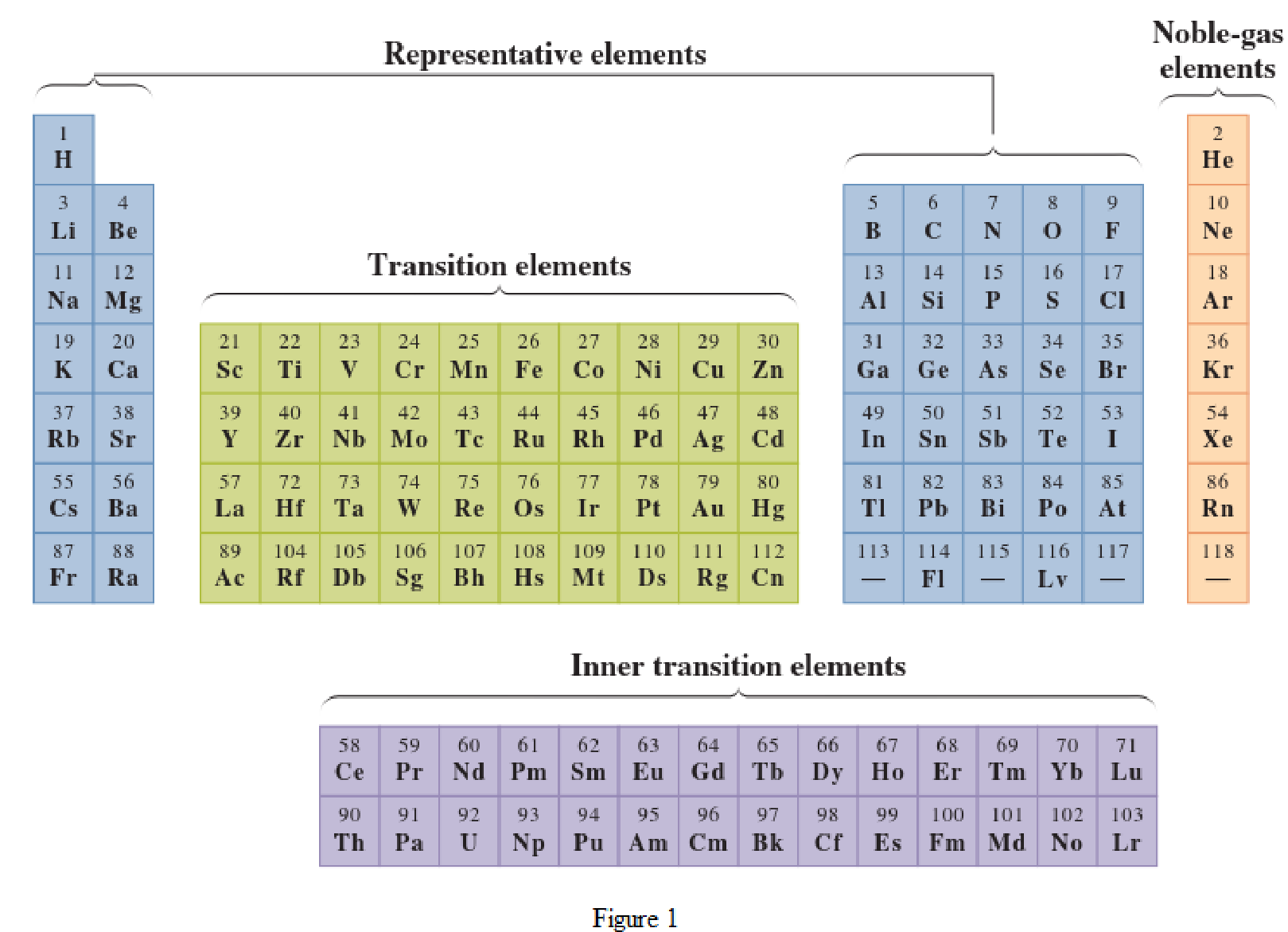
In the given periodic table, how many elements those are highlighted which represent transition have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(c)
Interpretation:
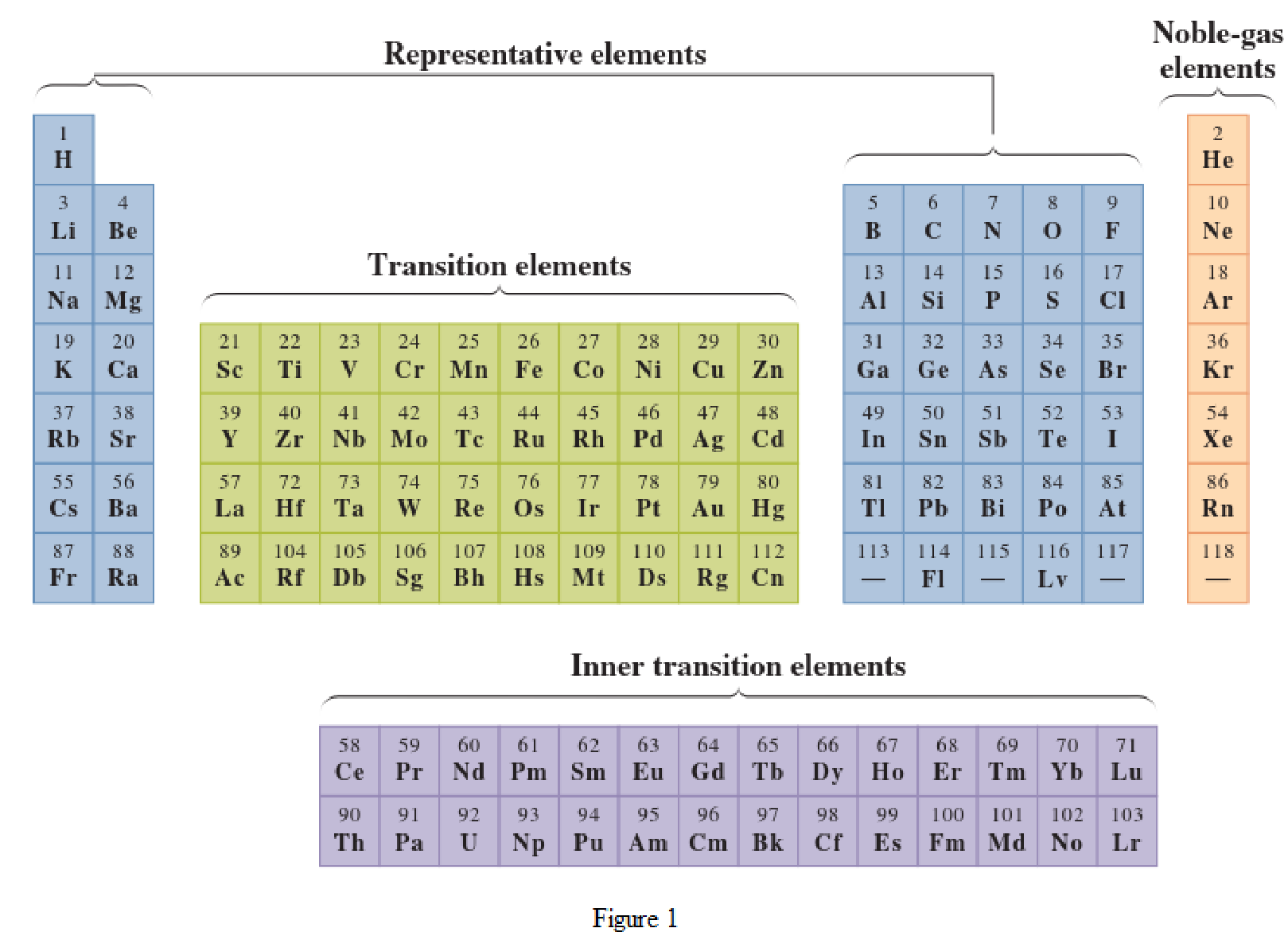
In the given periodic table, how many elements those are highlighted which represent metallic representative elements have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
(d)
Interpretation:
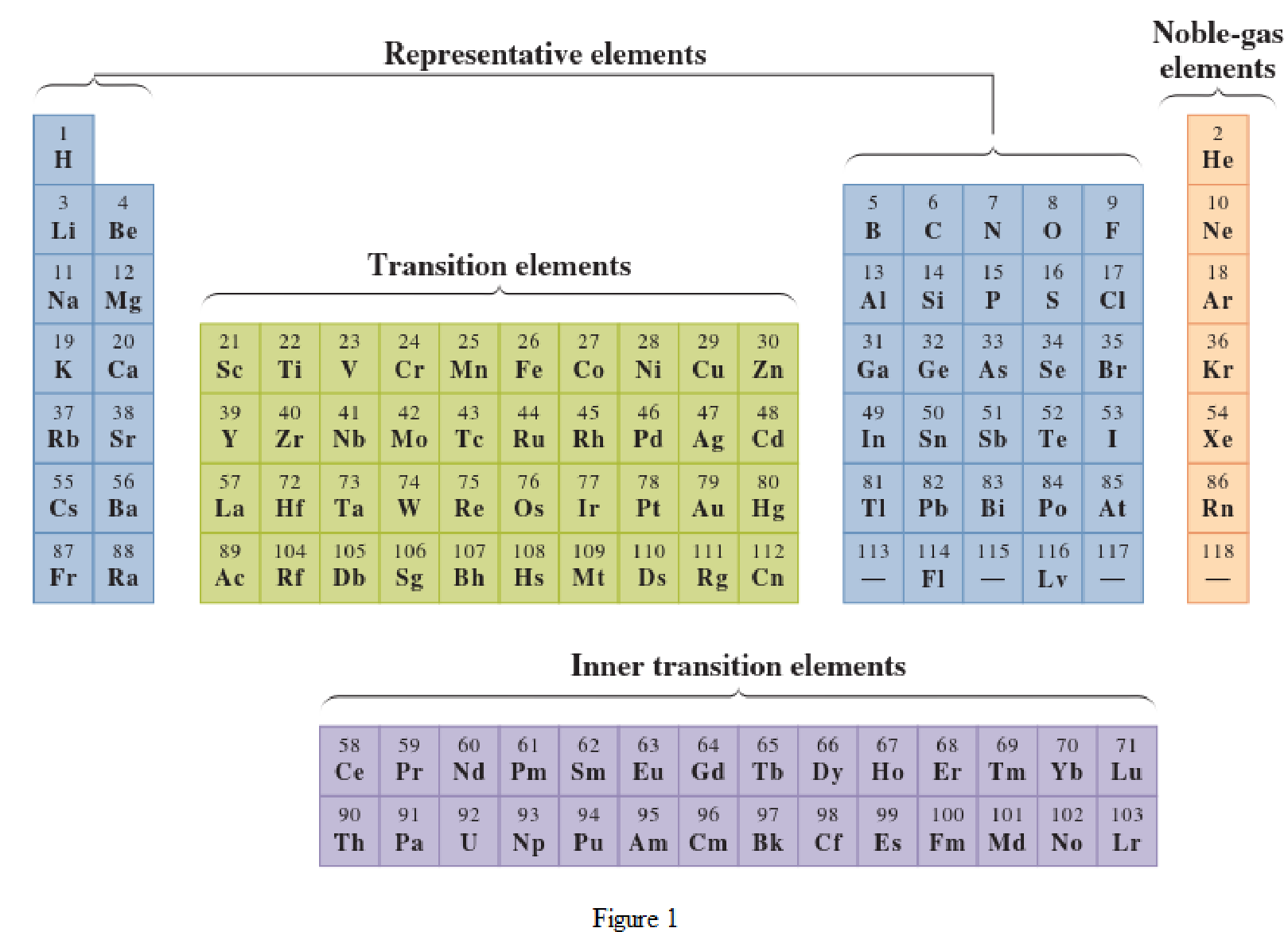
In the given periodic table, how many elements those are highlighted which represent nonmetals have to be determined.
Concept Introduction:
Elements in the periodic table are classified in several different ways and out of them two most common systems are,
- System based on the physical properties in which they are classified as metals and nonmetals.
- System based on electronic configuration in which they are classified as noble-gas, representative elements, transition elements, or inner-transition elements.
Noble-gas elements are the ones that are located in far right of periodic table. The physical state of these elements at room temperature is gas. The noble gases have their electronic configuration ending with
Representative elements are the ones that are in s area and area of the periodic table. They have partially filled s subshell or p subshell in their electronic configurations. Some of the elements are nonmetals while others are metals.
Transition elements are the ones that are located in d area of periodic table. They have the distinguishing electrons in their d subshell. All the transition elements are metals.
Inner transition elements are the ones that are located in f area of the periodic table. They have the distinguishing electrons in their f subshell. All inner transition elements are metals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
General, Organic, and Biological Chemistry
- Using the information given in the table in Problem 3-35 indicate whether each of the following pairs of atoms are isotopes. a. Atom B and atom C b. Atom B and atom D c. Atom C and atom Darrow_forwardAn ion that has two more electrons outside the nucleus than there are protons in the nucleus will have a charge of_________.arrow_forward2-102 An element consists of 90.51% of an isotope with a mass of 19.992 amu, 0.27% of an isotope with a mass of 20.994 amu, and 9.22% of an isotope with a mass of 21.990 amu. Calculate the average atomic mass and identify the element.arrow_forward
- 2.26 In what region of the periodic table are you likely to find elements that form more than one stable ion?arrow_forward2-101 Complete the following table: Symbol Atomic number Atomic weight Mass number # of protons # of neutrons # of electrons H 0 Li 4 3 Al 26 58 78 17 20arrow_forwardGive the symbol for an element that is: a a halogen; b an alkali metal; c a noble gas; d an alkaline earth metal.arrow_forward
- 2.84 Early attempts to arrange the elements often focused on atomic weight. Mendeleev considered a number of properties in addition to atomic weight, so he realized that some elements seemed out of place when ordered by atomic weight. Using the modern periodic table, identify elements for which Mendeleev must have had to switch the order in order to get the correct sequence of elements.arrow_forward2.42 What is a period in the periodic table? From what does it derive its name?arrow_forwardFill in the blanks to complete the following table. Row Element Name Element Symbol Atomic Number 1 -- AgAg 47 2 Tin -- -- 3 -- SeSe -- 4 Cobalt -- 27 5 -- CrCr -- 6 -- -- 46 Part A Fill in the blanks of the Element Name column of the table. Enter element names separated by commas. Enter your answers in order from the top to the bottom of the table. Element Name rows 1, 3, 5, 6 = Element Symbol rows 2, 4, 6= Atomic Number rows 2, 3, 5 =arrow_forward
- Review items 1 to 6 and answer questions a and b: The activities were designed to produce an EX4 research formulation. But not In the process, a mixture is supplied which also contains EX2. looking for features of these substances and their elements, obtained the following information: 1- Element E and element X have, respectively, four and seven electrons valence. 2- The elementary forms of E and X are, respectively, E(s) and X2(g). 3- The melting and boiling points of EX2 are, respectively, -50 and 10 °C. 4- The melting and boiling points of EX4 are, respectively, -70 and 20 °C. 5- For the compounds in question, the binding energy is lower in the situation where central atom (E) hybridization involves fewer orbitals. 6- There is heat release when 1 mol of each compound is formed from the related elementary forms. Based on this information, answer: a) Considering that the binding energy is influenced by electrostatic aspects, explain the difference in the binding energy of the EX. b)…arrow_forwardWhat is the element based on this clue: It has 3 shells of e- and 2e- in last shell?arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





