
Interpretation:
The suprafacial or antarafacial stereochemistry of the following sigmatropic reaction by order [x, y] is,
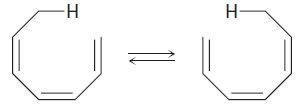
Concept introduction:
A sigmatropic rearrangement is a process in which a sigma-bonded substituent atom or group migrates across a pie electron system from one position to another. So, A sigmatropic reaction in organic chemistry is a pericyclic reaction wherein the net result is one σ-bond is changed to another σ-bond.
Sigmatropic rearrangements are concisely described by an order term [i, j], which is defined as the migration of a σ-bond adjacent to one or more π systems to a new position (i-1) and (j-1) atoms removed from the original location of the σ-bond. [3] When the sum of i and j is an even number, this is an indication of the involvement of a neutral, all C atom chain. An odd number is an indication of the involvement of a charged C atom or of a heteroatom lone pair replacing a C = C double bond. Thus, [1, 5] and [3, 3] shifts become [1, 4] and [2, 3] shifts with heteroatoms, while preserving symmetry considerations.
If the migrating group remains on the same face of the π system, the shift is known as suprafacial, while if the migrating group transfers to the opposite face is called an antarafacial shift.
Trending nowThis is a popular solution!

Chapter 30 Solutions
Organic Chemistry
- The bicyclic alkene P can be prepared by thermal electrocyclic ringclosure from cyclodecadiene Q or by photochemical electrocyclic ringclosure from cyclodecadiene R. Draw the structures of Q and R, andindicate the stereochemistry of the process by which each reactionoccurs.arrow_forwardOptically active (R)-2-bromobutane can be converted to 2-butanol under either conditions A or conditions B. Describe the stereochemistry of the product solutions for the two different conditions.arrow_forwardPredict the products and include stereochemistry.arrow_forward
- 1. (1-bromo-1, 3-dimethylcyclopentane) an optically active pure sample reacted with water. Write the complete mechanism which includes the electron-pushing arrows. Show the expected stereochemistry for each of the case and each mechanism should give two distinct products and then describe the relationship of the products to one another.arrow_forwardWhen 1,2-dimethylcyclopentene undergoes hydroboration–oxidation, one diastereomerof the product predominates. Show why this addition is stereospecific, and predict thestereochemistry of the major product.arrow_forwardThe transformation takes place via two sequential pericyclic reactions. Identify the two reactions and give a critical explanation whether the reactions are allowable or not. Explain the stereochemistry.arrow_forward
- Rank the following compounds in order of their expected reactivity towardSN2 reactions and explain your order. CH3Br, CH3OToS, CH3CCl, CH3CHClarrow_forwardWhich of the following reaction coordinate diagrams represents SN1 and E1 reactions? A B C Darrow_forwardExplain the Stereochemistry of the SN2 Reaction ?arrow_forward
- For each one of the following reactions, show step by step mechanism of the reactions and indicate the stereochemistry of the products and their relationshiparrow_forwardElimination occurs when (Z)-3-bromohex-3-ene is treated with NaNH2. Under the same conditions, 1-bromocyclohexeneundergoes elimination much more sluggishly. Explain whyarrow_forwarda) What product is formed from the [1,7] sigmatropic rearrangement of a deuterium in the following triene? (b) Does this reaction proceed in a suprafacial or antarafacial manner under thermal conditions? (c) Does this reaction proceed in a suprafacial or antarafacial manner under photochemical conditions?arrow_forward
