
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 30.8, Problem 9P
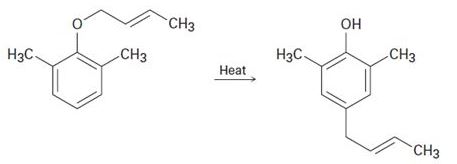
When a 2, 6-disubstituted allyl phenyl ether is heated in an attempted Claisen rearrangement, migration occurs to give the p-allyl product as the result of two sequential pericyclic reactions. Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
What are the products of the following ether cleavage reactions? What is the mechanism that follows?
Provide the missing reagents and products for each transformation shown below.
Q2: Give the structure of the possible Claisen condensation product from the following reaction .Tell which , If any you would expect to predominate in each case .(a) CH3CO2Et + CH3CH2CO2Et (b) C6H6CO2Et + C6H5CH2CO2Et(c) EtOCO2Et + Cyclohexanones (d) C6H5CHO + CH3CO2Et
Chapter 30 Solutions
Organic Chemistry
Ch. 30.1 - Prob. 1PCh. 30.3 - Prob. 2PCh. 30.3 - Prob. 3PCh. 30.4 - Prob. 4PCh. 30.6 - What stereochemistry would you expect for the...Ch. 30.6 - Prob. 6PCh. 30.7 - Prob. 7PCh. 30.8 - Propose a mechanism to account for the fact that...Ch. 30.8 - When a 2, 6-disubstituted allyl phenyl ether is...Ch. 30.9 - Prob. 10P
Ch. 30.SE - Predict the product obtained when the following...Ch. 30.SE - Prob. 12VCCh. 30.SE - The following rearrangement of N-allyl-N,...Ch. 30.SE - Plastic photochromic sunglasses are based on the...Ch. 30.SE - Prob. 15MPCh. 30.SE - Prob. 16MPCh. 30.SE - Prob. 17MPCh. 30.SE - Prob. 18APCh. 30.SE - Prob. 19APCh. 30.SE - Prob. 20APCh. 30.SE - Prob. 21APCh. 30.SE - Prob. 22APCh. 30.SE - Prob. 23APCh. 30.SE - Prob. 24APCh. 30.SE - Prob. 25APCh. 30.SE - Prob. 26APCh. 30.SE - Prob. 27APCh. 30.SE - Prob. 28APCh. 30.SE - Propose a pericyclic mechanism to account for the...Ch. 30.SE - Prob. 30APCh. 30.SE - Prob. 31APCh. 30.SE - Prob. 32APCh. 30.SE - Prob. 33APCh. 30.SE - Bicyclohexadiene, also known as Dewar benzene, is...Ch. 30.SE - Prob. 35APCh. 30.SE - Prob. 36APCh. 30.SE - The 1H NMR spectrum of bullvalene at 100 C...Ch. 30.SE - Prob. 38APCh. 30.SE - Prob. 39APCh. 30.SE - Prob. 40APCh. 30.SE - In light of your answer to Problem 30-40, explain...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the major product of the following reaction sequence?Tek seçenek.arrow_forwardThese reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4arrow_forwardAs we will learn in Chapter 9, an epoxide is an ether with an oxygen atom in a three-membered ring. Epoxides can be made by intramolecular SN2 reactions of intermediates that contain a nucleophile and a leaving group on adjacent carbons, as shown.Assume that each of the following starting materials can be converted to an epoxide by this reaction. Draw the product formed (including stereochemistry) from each starting material. Why might some of these reactions be more difficult than others in yielding nucleophilic substitution products?arrow_forward
- Complete the following reactions by providing the missing product(s). Determine what mechanism operates in each case (SN1, SN2, E1, or E2). Show the stereochemistry of the product(s) where appropriate. If more than one product is formed circle the major one.arrow_forwardA chemist requires a large amount of 1-bromo-5-methyl-2-hexene as starting material for a synthesis and decides to carry out the following NBS allylic bromination reaction in the presence of UV light. Draw the structures of all of the observed products.arrow_forwardA) what is the major product if E2 mechanism is followed in the reaction (A-D) B) what is the complete mechanism with steps to form the major productarrow_forward
- Given that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5 −is a phenyl group, a benzene ring bonded to another group.arrow_forwardGiven that an E2 reaction proceeds with anti periplanar stereochemistry, draw the products of each elimination. The alkyl halides in (a) and (b) are diastereomers of each other. How are the products of these two reactions related? Recall from Section 3.2A that C6H5– is a phenyl group, a benzene ring bonded to another group.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY