
Concept explainers
a)
Interpretation: Positive or negative sign above curved arrow to indicate sign of energy change associated with the below process should be determined.
Concept introduction:
b)
Interpretation: Whether bond breakage in
Concept introduction: Chemical reactions that require absorption of energy for their occurrence are known as endothermic reactions. But chemical reactions that are accompanied by the release of energy are known as exothermic reactions. For example, the melting of ice is an example of an endothermic reaction as energy is provided for this process. The reaction between water and calcium chloride is an example of an exothermic reaction.
c)
Interpretation: Whether arrow is more likely to be associated with below left or below right figure should be determined.
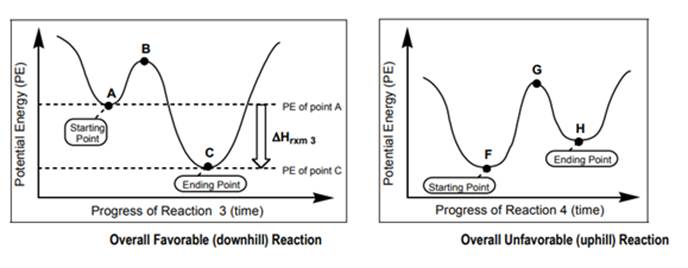
Concept introduction: Chemical reactions that require absorption of energy for their occurrence are known as endothermic reactions. But chemical reactions that are accompanied by the release of energy are known as exothermic reactions. For example, the melting of ice is an example of an endothermic reaction as energy is provided for this process. The reaction between water and calcium chloride is an example of an exothermic reaction.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Custom eBook for Organic Chemistry
- What is the order of the punch and the energy diagram according to the T.O.M molecular theory of F2?arrow_forwardI have difficulty understanding the second part of the question. Please answer all parts and explain F and G in detail. thank youarrow_forward13. Aniline and nitrobenzene are two substituted benzene compounds that contain nitrogen atoms. A) Use resonance structures to identify all carbon atoms that are electron rich on aniline. Mark the appropriate carbons in the figure below with a d-. NH₂ nitrobenzene aniline Note: this is benzene B) Use resonance structures to identify all carbon atoms that are electron deficient on nitrobenzene. Mark the appropriate carbons in the figure below with a dª.arrow_forward
- 9. The two molecules shown below are structurally very similar. Draw all resonance structure for each species with curved arrows and circle which is molecule (A or B) is more stable because it has more resonance structures. Only use patterns 2 and 3. A Barrow_forward! ( do b with explanation)arrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. What is the relationship between:a. I and II? III and IV?b. I and IV? II and IV?c. I and III? II and III?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
