
Concept explainers
(a)
Interpretation:
The Lewis structure for an aldetetrose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. The general formula for monosaccharides is
Answer to Problem 4.25P
The Lewis structure for an aldetetrose is
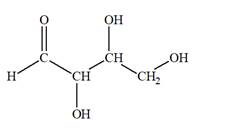
Explanation of Solution
Aldotetrose is a mono-saccharide. The name “aldo” indicates that the carbonyl group must be present at the terminal carbon atom, while the word “tetrose” indicates a chain of four carbon atoms. Thus, aldotetrose is a sugar having an
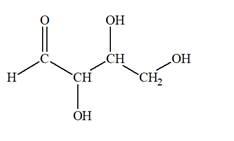
The molecular formula for the above structure is
Altetrose is a mono-saccharide having a carbonyl group at the terminal carbon atom, and its molecular formula is
(b)
Interpretation:
The Lewis structure for a ketotetrose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. The general formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketotetrose is
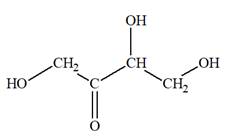
Explanation of Solution
Ketotetrose is a type of mono-saccharide. The name “keto” indicates that the carbonyl group must be present at the internal carbon atom, while the word “tetrose” indicates the chain of four carbon atoms. Thus, ketotetrose is a sugar having a carbonyl group at the internal carbon atom, and the structure must have four carbon atoms. The general molecular formula for mono-saccharides is
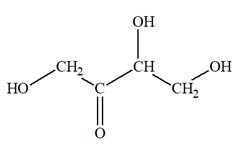
The molecular formula for the above structure is
Ketotetrose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
(c)
Interpretation:
The Lewis structure for an aldetriose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for an aldetriose is
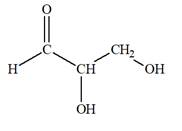
Explanation of Solution
Aldotriose is a type of mono-saccharide. The name “aldo”’ indicates that the carbonyl group must be present at the terminal carbon atom, while the word “triose” indicates a chain of three carbon atoms. Thus, aldotriose is a sugar having an aldehyde group at the terminal carbon atom, and the structure must have three carbon chain. The general molecular formula for mono-saccharides is

The molecular formula for the above structure is
Altetrose is a mono-saccharide having a carbonyl group at the terminal carbon atom, and its molecular formula is
(d)
Interpretation:
The Lewis structure for a ketotriose is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketotriose is
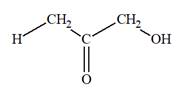
Explanation of Solution
Ketotriose is a type of mono-saccharide. The name “keto” indicates that the carbonyl group must be present at the internal carbon atom, while the word “tetrose” indicates the chain of four carbon atoms. Thus, ketotriose is a sugar having a carbonyl group at the internal carbon atom, and the structure must have three carbon atoms. The general molecular formula for mono-saccharides is

The molecular formula for the above structure is
Ketotetrose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
(e)
Interpretation:
The Lewis structure for a ketohexose, which is different from fructose, is to be drawn.
Concept introduction:
Monosaccharides are called simple sugars, with the number of oxygen atoms same as the number of carbon atoms. Thus, the general molecular formula for a monosaccharide is
Answer to Problem 4.25P
The Lewis structure for a ketohexose, which is different from fructose, is
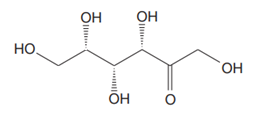
Explanation of Solution
The Lewis structure for fructose is
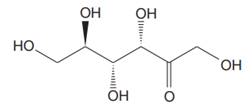
Fructose is a ketohexose with a molecular formula

The molecular formula for the above structure is
Ketohexose is a mono-saccharide having a carbonyl group at the internal carbon atom, and its molecular formula is
Want to see more full solutions like this?
Chapter 4 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- The chiral molecular among the following isarrow_forwardRubber degrades when it is exposed to ozone for an extended time— a phenomenon called ozone cracking. Rubber is a natural polymer (a long-chain molecule with a regular repeating structural unit), a portion of whichis shown here. Explain why ozone cracking occursarrow_forwardAnswer with explanation Asap identify the functional groupsarrow_forward
- Wool, silk, acetate, cotton, nylon, dacron, orlon Which type of fiber(s) is/are likely to be the most polar? Explain. Which type of fiber(s) is/are likely to be the least polar? Explain.arrow_forwardPut the lone pairs on the following structures where appropriate and draw the curved arrows on the left-hand structures that will result in the right-hand structures.arrow_forwardplease show which type of arrows are used. since there is a double bond arrow or single bond arrow. you can see it in the picture.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

