
Concept explainers
(a)
Interpretation:
IHD for the compound having the molecular formula
Concept introduction:
In order to determine the IHD of a given molecular formula, first draw any saturated molecule that has the same number of each non-hydrogen atom as in the given formula. The general formula of a saturated hydrocarbon is
Answer to Problem 4.49P
IHD for the compound having molecular formula
Explanation of Solution
The given molecular formula is
Thus, this saturated molecule has 8 additional hydrogen atoms as compared to the given molecular formula. IHD for the given molecular formula is calculated by dividing that number of additional hydrogen atoms by 2. Thus, IHD is
The IHD for the compound with a given molecular formula is calculated by applying the steps above.
(b)
Interpretation:
IHD for the compound having the molecular formula
Concept introduction:
In order to determine the IHD of a given molecular formula, first draw any saturated molecule that has the same number of each non-hydrogen atom as in the given formula. The general formula of a saturated hydrocarbon is
Answer to Problem 4.49P
IHD for the compound having molecular formula
Explanation of Solution
The given molecular formula is
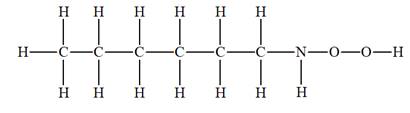
It takes a total of 15 hydrogen atoms to saturate each carbon, nitrogen, and oxygen in this compound. Thus, this saturated molecule has 10 additional hydrogen atoms as compared to the given molecular formula. IHD for the given molecular formula is calculated by dividing that number of additional hydrogen atoms by 2. Thus IHD is
The IHD for the compound with a given molecular formula is calculated by applying the steps above.
(c)
Interpretation:
IHD for the compound having the molecular formula
Concept introduction:
In order to determine the IHD of a given molecular formula, first draw any saturated molecule that has the same number of each non-hydrogen atom as in the given formula. The general formula of a saturated hydrocarbon is
Answer to Problem 4.49P
IHD for the compound having molecular formula
Explanation of Solution
The given molecular formula is
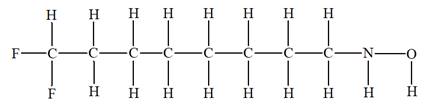
It takes a total of
The IHD for the compound with a given molecular formula is calculated by applying the steps above.
(d)
Interpretation:
IHD for the compound having the molecular formula
Concept introduction:
In order to determine the IHD of a given molecular formula, first draw any saturated molecule that has the same number of each non-hydrogen atom as in the given formula. The general formula of a saturated hydrocarbon is
Answer to Problem 4.49P
IHD for the compound having molecular formula
Explanation of Solution
The given molecular formula is
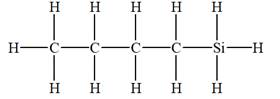
It takes a total of
The IHD for the compound with a given molecular formula is calculated by applying the steps above.
(f)
Interpretation:
IHD for the compound having the molecular formula
Concept introduction:
In order to determine the IHD of a given molecular formula, first draw any saturated molecule that has the same number of each non-hydrogen atom as in the given formula. The general formula of a saturated hydrocarbon is
Answer to Problem 4.49P
IHD for the compound having molecular formula
Explanation of Solution
The given molecular formula is
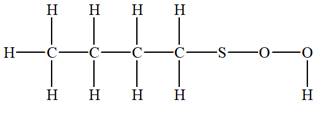
It takes a total of
The IHD for the compound with a given molecular formula is calculated by applying the steps above.
Want to see more full solutions like this?
Chapter 4 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- A student draws the picture of ammonia (NH3) in the box below, left, and predicts it will be a flatmolecule with HNH bond angles of exactly 120°. Unfortunately, the student left something out. a. What did the student omit from his drawing? b. What is the actual HNH bond angle of ammonia (based on the draw g above, right)? c. Explain why water, ammonia, and methane (shown below) all have about the same bondangles (close to 109.5°) even though they have different numbers of bonds.arrow_forwardAll carbon-to-carbon bond lengths are identical in benzene. Does this argue for or against the presence of C=C bonds in benzene? Explain.arrow_forwardConsider the following molecules: SiH4, PH3, H2S. In each case, a central atom is surrounded by four electron pairs. In which of these molecules would you expect the bond angle to be less than 109.5? Explain your reasoning.arrow_forward
- The following model is a representation of aspartame, C14H18N2O5, known commercially under many names, including NutraSweet. Only the connections between atoms are shown; multiple bonds are not indicated. Complete the structure for aspartame, and indicate the positions of multiple bonds (gray = C, red = O, blue = N, ivory = H).arrow_forwardFollowing are the structures of three isomers of difluorobenzene, C6H4F2. Are any of them nonpolar?arrow_forwardThe cations O2+ and N2+ are formed when molecules of O2 and N2 are subjected to intense, high-energy solar radiation in Earths upper atmosphere. Write the electron configuration for O2+. Predict its bond order and magnetic behavior.arrow_forward
- Write molecular formulas for the five possible molecular ions of m/z 100 containing only the elements C, H, N, and O.arrow_forwardA molecule of formula AY₃ is found experimentally to bepolar. Which molecular shapes are possible and which impossi-ble for AY₃?arrow_forwardDraw PF3 and PH3. Are they polar and do their polarities differ?arrow_forward
- Methyl isocyanate, CH3 -N= C = O, is used in the industrial synthesis of a type of pesticide and herbicide known as a carbamate. As a historical note, an industrial accident in Bhopal, India, in 1984 resulted in leakage of an unknown quantity of this chemical into the air. An estimated 200,000 people were exposed to its vapors, and over 2000 of these people died. Q.) Write a Lewis structure for methyl isocyanate and predict its bond angles. What is the hybridization of its carbonyl carbon? Of its nitrogen atom?arrow_forwardHow many different structural isomers are there for octahedral molecules with the general formula AX2Y3 if A is the inner atom and there are no X-Y, X-X and Y-Y bonds?arrow_forwardWRITE THE COMPLETE COMPUTATION of the FORMAL CHARGE of each atom using the formula: FC = V - N - B/2 a. Ethanol b. Chlorate ionarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





