
Concept explainers
(a)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is zero.
Explanation of Solution
The given compound is
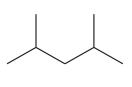
This compound has all single bonds and is a saturated compound. There are no double bonds, triple bonds, or rings in the molecule. Each carbon atom is bonded to four other atoms via single bonds. Thus, the index of hydrogen deficiency for this compound is zero.
The index of hydrogen deficiency for a saturated compound is zero.
(b)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is one.
Explanation of Solution
The given compound is
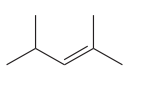
The given compound has one double bond in its structure. Each double bond contributes 1 to the index of hydrogen deficiency. The given structure does not contain triple bonds and rings. Thus, for this compound, the index of hydrogen deficiency is 1.
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(c)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is three.
Explanation of Solution
The given compound is
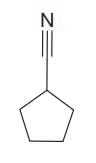
The given compound has one triple bond in its structure. Each triple bond contributes 2 to the index of hydrogen deficiency. There is one ring in the structure. Each ring contributes 1 to the index of hydrogen deficiency. Thus, for this compound, the index of hydrogen deficiency is
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(d)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is four.
Explanation of Solution
The given compound is
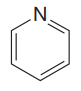
The given compound has one ring in its structure. Each ring contributes 1 to the index of hydrogen deficiency. There are three double bonds in the structure. Each double bond contributes 1 to the index of hydrogen deficiency. Thus, the index of hydrogen deficiency for this compound is
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(e)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is one.
Explanation of Solution
The given compound is
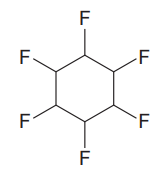
The given compound has one ring in its structure. Each ring contributes 1 to the index of hydrogen deficiency. There are no double bonds and triple bonds in the structure. Thus, the index of hydrogen deficiency for this compound is 1.
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(f)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is four.
Explanation of Solution
The given compound is
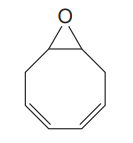
The given compound has two rings in its structure. Each ring contributes 1 to the index of hydrogen deficiency. There are two double bonds in the structure. Each double bond contributes 1 to the index of hydrogen deficiency. Thus, the index of hydrogen deficiency for this compound is
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(g)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is five.
Explanation of Solution
The given compound is
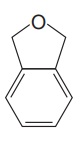
The given compound has two rings in its structure. Each ring contributes 1 to the index of hydrogen deficiency. There are three double bonds in the structure. Each double bond contributes 1 to the index of hydrogen deficiency. Thus, the index of hydrogen deficiency for this compound is
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
(h)
Interpretation:
The index of hydrogen deficiency for the given compound is to be determined.
Concept introduction:
The index of hydrogen deficiency of a molecule is the extent to which the molecule is unsaturated. It is half the number of hydrogen atoms missing from the molecule as compared to a completely saturated molecule. The contribution of each double bond in a molecule to the molecule’s index of hydrogen deficiency is 1. The contribution of each triple bond in a molecule to the molecule’s index of hydrogen deficiency is 2. The contribution of each ring in a molecule to the molecule’s index of hydrogen deficiency is 1. If a molecule is saturated and has no rings, double bonds, and triple bonds, its index of hydrogen deficiency is 0.
Answer to Problem 4.47P
The index of hydrogen deficiency for the given compound is five.
Explanation of Solution
The given compound is
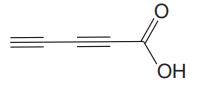
The given compound has no rings in its structure. There is one double bond in the structure. Each double bond contributes 1 to the index of hydrogen deficiency. There are two triple bonds in the structure. Each triple bond contributes 2 to the index of hydrogen deficiency. Thus, the index of hydrogen deficiency for this compound is
The index of hydrogen deficiency for an unsaturated compound depends on the number of double bonds, triple bonds, and rings in its structure.
Want to see more full solutions like this?
Chapter 4 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Are my chair conformations correct? What about the energies of each conformation according to the table?arrow_forwardTotal number of stereoisomers of the compound given in the attached question.arrow_forward(select lowest enegy conformation do all ) ( please answer with explantion for both correct and incorrect option ) do allarrow_forward
- Draw only the diastereomer(s) of the following moleculearrow_forwardHelp with the following question. Please round the answer to 2 sig figsarrow_forwardConsider the molecule 1-bromo-2-methylbutane. C3 and C4 should be drawn as Et as in theexample. This group is called an ethyl group and can be considered a sphere about twice the sizeof a methyl group. Draw the following Newman projections sighting down the C1C2 bond... a. The lowest potential energy conformation. b. The highest potential energy staggered conformation.arrow_forward
- Draw a Newman projection showing the lowest P.E. conformation of the following moleculesighting down the C2C3 bond (as indicated below). Show methyl and ethyl groups as Me and Et.arrow_forwardIdentify circle the alkanelike portions of the following molecules: a. b. c. d.arrow_forwardDraw the alternate conformation of the structure shown below.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

