
The ingenious Stirling engine is a true
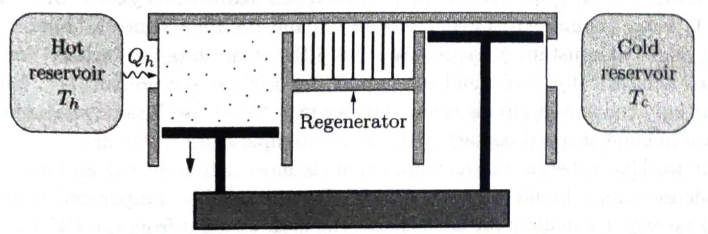
Figure 4.7. A Stirling engine, shown during the power stroke when the hot piston is moving outward and the cold piston is at rest. (For simplicity, the linkages between the two pistons are not shown.)
Trending nowThis is a popular solution!

Chapter 4 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Physics (5th Edition)
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Applied Physics (11th Edition)
Life in the Universe (4th Edition)
Physics for Scientists and Engineers with Modern Physics
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
- please do fast 5. Find the expression for the entropy of a single harmonic oscillator.arrow_forwardWhat are the key principles and applications of the adiabatic approximation in the field of physics?arrow_forwardWhy do the collisions between molecules do not appear in the derivation of the ideal gas law?arrow_forward
- Under what conditions is the ideal-gas assumption suitable for real gases?arrow_forwardAn engineer claims to have measured the characteristics of a heat engine that takes in 100 J of thermal energy and produces 50 J of useful work. Is this engine possible? If so, what is the smallest possible ratio of the temperatures (in kelvin) of the hot and cold reservoirs?arrow_forwardIf you have 9 moles of a monoatomic ideal gas, how much heat is required to raise the temperature of this gas from 264.3K to 277.6K if the volume of the gas remains constant during the heating? Note: It is understood that your answer is in units of Joulesarrow_forward
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





