
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4.7, Problem 4.63P
Interpretation Introduction
Interpretation:
The positions that are prone to electrophilic
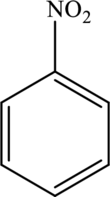
Concept Introduction:
Deactivators are electron withdrawing groups attached to the benzenes that have either positive charge or an atom with high electronegativity. They are meta directors.
- Strong deactivators: They are very strong electron withdrawing groups.
- Moderate deactivators: It contains a pi bond that is attached to a strong electronegative group.
- Weak deactivators: It consists of halogens.
Activators are electron donating groups attached to the benzenes that have either electron density that is able to push into benzene ring or a lone pair of electrons. They are ortho-para directing.
- Strong activators: It contains a lone pair next to the aromatic ring.
- Moderate activators: It has a lone pair next to the aromatic ring that can take part in the resonance outside the ring as well.
- Weak activators: It consists of alkyl groups.
Halogens are deactivators that are ortho-para directing.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For an ester, which absorbance would one expect to observe?
Group of answer choices
1150-1350
1000-1260
1210-1320
1715-1740
1000-1150
Infrared spectrum: interpret all absorptions from the 4000-1400 cm-1 region of the spectrum. Indicate which absorptions come from the ester and which from impurities. Submit the spectrum with your report.
Which of the following molecules could not be used to make an amide directly with ammonia? (Circle all that apply.)
Time Left: 00:09:40
a) Ethanoic acid
b) Ethanoic anhydride
c) Ethyl ethancate
d Ethanonitrile
e) Ethanoyl chloride
Chapter 4 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 4.1 - Consider the following reaction, in which an...Ch. 4.1 - Prob. 4.3PCh. 4.1 - Aromatic rings will also undergo iodination when...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.2 - In each of the following cases, identify the...Ch. 4.3 - Prob. 4.10PCh. 4.3 - Prob. 4.11PCh. 4.3 - Prob. 4.12PCh. 4.3 - Prob. 4.13P
Ch. 4.3 - Prob. 4.14PCh. 4.3 - Predict the products of the following reaction.Ch. 4.3 - Prob. 4.16PCh. 4.3 - Prob. 4.17PCh. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - Identify the reagents you would use to achieve...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - fill in the reagents you would use for the...Ch. 4.4 - Prob. 4.27PCh. 4.4 - Prob. 4.28PCh. 4.4 - And now, for a challenging problem, try to draw...Ch. 4.6 - Prob. 4.31PCh. 4.6 - Prob. 4.32PCh. 4.6 - Prob. 4.33PCh. 4.6 - Prob. 4.34PCh. 4.6 - Prob. 4.35PCh. 4.6 - Prob. 4.36PCh. 4.6 - Prob. 4.37PCh. 4.6 - Prob. 4.40PCh. 4.6 - Prob. 4.41PCh. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Predict the products for each of the following...Ch. 4.6 - Prob. 4.47PCh. 4.6 - Prob. 4.48PCh. 4.6 - Prob. 4.49PCh. 4.6 - Prob. 4.50PCh. 4.6 - Prob. 4.51PCh. 4.6 - Prob. 4.52PCh. 4.6 - Prob. 4.53PCh. 4.6 - Prob. 4.54PCh. 4.6 - Prob. 4.55PCh. 4.6 - Prob. 4.56PCh. 4.7 - Prob. 4.58PCh. 4.7 - Prob. 4.59PCh. 4.7 - Prob. 4.60PCh. 4.7 - Prob. 4.61PCh. 4.7 - Prob. 4.62PCh. 4.7 - Prob. 4.63PCh. 4.7 - Prob. 4.64PCh. 4.7 - Prob. 4.65PCh. 4.7 - Prob. 4.66PCh. 4.7 - Prob. 4.67PCh. 4.7 - Can you explain why the following group is a...Ch. 4.7 - Prob. 4.70PCh. 4.7 - Prob. 4.71PCh. 4.7 - Prob. 4.72PCh. 4.7 - Prob. 4.73PCh. 4.7 - Prob. 4.74PCh. 4.7 - Prob. 4.76PCh. 4.7 - Prob. 4.77PCh. 4.7 - Prob. 4.78PCh. 4.7 - Prob. 4.79PCh. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Propose an efficient synthesis for each of the...Ch. 4.8 - Prob. 4.87PCh. 4.8 - Prob. 4.88PCh. 4.8 - Prob. 4.89PCh. 4.8 - Prob. 4.90PCh. 4.8 - Prob. 4.91PCh. 4.8 - Prob. 4.92PCh. 4.9 - Prob. 4.94PCh. 4.9 - Prob. 4.95PCh. 4.9 - Prob. 4.96PCh. 4.9 - Prob. 4.97PCh. 4.9 - Prob. 4.98PCh. 4.9 - Prob. 4.99PCh. 4.9 - Prob. 4.100PCh. 4.9 - Prob. 4.101PCh. 4.9 - Prob. 4.102P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Considering IR spectroscopy, which of the following statements is incorrect? Question 34 options: Symmetrical C=C bonds and C≡C bonds give signals at lower wavenumbers compared to unsymmetrical bonds Primary amines produce two signals resulting from symmetric stretching and asymmetric stretching Carboxylic acids exist as dimer due to hydrogen bonding and thus produce a broad signal at 2200-3600 cm-1 and a characteristic signal due to C=O at approx. 1700 cm-1 Concentrated alcohols give rise to broad signals while dilute alcohols give rise to narrow signals C=O bonds produce strong signals in an IR spectrum while C=C bonds often produce a weak signalsarrow_forward1. (a) Identify all transfers by arrows (P-transfer or N-attach) also identify nucleophiles and electrophiles, bases, and acids. (b) at the bottom page draw the net reaction going from “imine” to “conjugate acid of the aldehyde”. I only need help with part b.arrow_forwardpost lab 1 Why is the acetamide group a weaker activating group than the amino group itself in an electrophilic aromatic substitution?arrow_forward
- Amines Instruction: Propose a synthesis pathway for the following. 1. benzoic acid to benzylamine 2. benzene carboxamide to benzylaminearrow_forwardCompound MM (g/mol) ortho mp (oC) meta mp (oC) para mp (oC) Nitroacetanilide 180.16 94 155 214-217 Methyl nitrobenzoate 181.15 -13 78-80 94-96 Based on the table above, determine the regiochemistry of your methyl nitrobenzoate and draw the structure Based on the table above , determine the regiochemistry of your nitroacetanilide and draw the structure.arrow_forwardbased on resonance and inductive effects, identify the strongest base among the aniline derivatives shown below aktiv please answer asaparrow_forward
- Identify which structural effect predominantly accounts for each given observation or phenomenon. Choose your answers from the following: A = Resonance B = C-H hyperconjugation C = Inductive Effect D = Steric Effect Meta-fluorophenol is a stronger acid than phenol.arrow_forwardWhich of the following class of amines will not have an NH absorption in its IR spectrum or an NH signal on its proton NMR, but will still have an odd-numbered M+? primary secondary tertiary all of these will have an NH IR absorbance and H-1 NMR signal (peak) Thank you!arrow_forwardWhen determining the identity of your unknown neutral product, you analysed it by IR. What feature(s) on your IR spectrum could help you distinguish piperonal from stilbene? Question 9 options: presence of sp2 C-H stretch near 2850 cm-1 presence of C=O stretch near 1700 cm-1 presence of C-O stretch near 1100 cm-1 more than one of the abovearrow_forward
- Which bases can deprotonate acetylene? The pKa values of the conjugate acids are given in parentheses. a. CH3NH− (pKa = 40) b. CO32− (pKa = 10.2) c.CH2=CH− (pKa = 44) d.(CH3)3CO− (pKa = 18)arrow_forward23- The NMR spectrum of the compound with formula C8HnN is shown. The spectrum in the infrared presents a doublet around 3350 cm'. Don't try to interpret the area of aromatic protons between 7.1 and 7.3 ppm, except to determine the number of protons attached to the aromatic ring. Draw the structure of the compound. note: point out the structures on the chart for better understandingarrow_forwardPost Lab Questions - Synthesis of Fluroscein 3.Explain why there are differences in absorbances (specifically wavelengths) of fluroscein and phenolphthalein. Which one would you expect to be a higher wavelength and why?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY