
Concept explainers
(a)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is enantiomers.
Explanation of Solution
The given pair of compounds is,
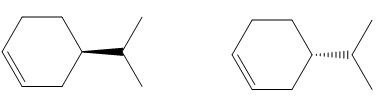
Figure 1
The given compounds have same connectivity of atoms or groups but have opposite configuration at the stereogenic centre. Hence, the given pair of compounds is enantiomers.
The given pair of compounds is enantiomers.
(b)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is constitutional isomers.
Explanation of Solution
The molecular formula of both given compounds is
The given pair of compounds is constitutional isomers.
(c)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is diastereomers.
Explanation of Solution
The given pair of compounds is,
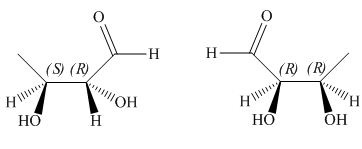
Figure 2
The given compounds have one stereogenic centre with same configuration and one with opposite configuration as shown in Figure 2. Hence, the given pair of compounds is diastereomers.
The given pair of compounds is diastereomers.
(d)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is not isomers of each other.
Explanation of Solution
The molecular formula of given compounds is different from the each other. The molecular formula of one is
The given pair of compounds is not isomers of each other.
(e)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is enantiomers.
Explanation of Solution
The given pair of compounds is,
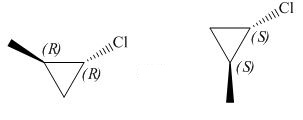
Figure 3
The given compounds have opposite configurations at the two stereogenic centre as shown in Figure 3. Hence, the given pair of compounds is enantiomers.
The given pair of compounds is enantiomers.
(f)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is enantiomers.
Explanation of Solution
The given pair of compounds is,
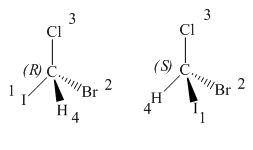
Figure 4
In the given compounds,
Thus, the two compounds have opposite configurations. Hence, the given pair of compounds is enantiomers.
The given pair of compounds is enantiomers.
(g)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is identical.
Explanation of Solution
The given pair of compounds is,
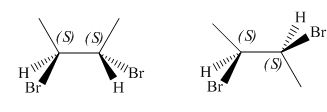
Figure 5
The given compounds have same connectivity and same configurations at the two stereogenic centre as shown in Figure 5. Hence, the given pair of compounds is identical.
The given pair of compounds is identical.
(h)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is diastereomers.
Explanation of Solution
The given pair of compounds is,
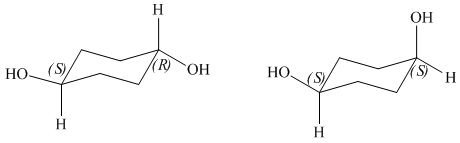
Figure 6
The given compounds have one stereogenic centre with same configuration and one with opposite configuration as shown in Figure 6. Hence, the given pair of compounds is diastereomers.
The given pair of compounds is diastereomers.
(i)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is diastereomers.
Explanation of Solution
The given pair of compounds is,
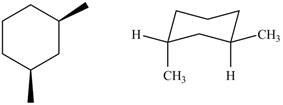
Figure 7
Two methyl groups present on the six membered ring are above the plane. However, the methyl groups of chair conformation are present trans to each other. Therefore, the given pair of compounds represents diastereomers.
The given pair of compounds is diastereomers.
(j)
Interpretation: The relation between given pair of compounds is to be determined.
Concept introduction: The stereogenic centers with
Enantiomer of a compound has opposite configuration at stereogenic centers. Diastereomers of a compound have at least one stereogenic centre with same configuration and at least one with opposite configuration.
Answer to Problem 5.62P
The given pair of compounds is constitutional isomers.
Explanation of Solution
The given pair of compounds is,
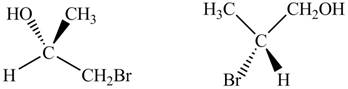
Figure 8
In the given compounds, the position of
The given pair of compounds is constitutional isomers.
Want to see more full solutions like this?
Chapter 5 Solutions
Organic Chemistry-Package(Custom)
- How is compound A related to compounds B–E? Choose from enantiomers, diastereomers, constitutional isomers, or identical molecules.arrow_forwardHow are the compounds in attached pair related to each other? Are they identical, enantiomers, diastereomers, constitutional isomers, or not isomers of each other??arrow_forwardLabel compounds B–D as stereoisomers, conformations, or constitutional isomers of Aarrow_forward
- Answer the following question about compounds A–D (See in attachment) How are the compounds in each pair related? Choose fromconstitutional isomers, stereoisomers, or identical molecules: A and B; A and C; B and D.arrow_forwardClassify each compound as identical to A or its enantiomer.arrow_forwardA is a toxin produced by the poisonous seaweed Chlorodesmis fastigiata. (a) Draw a stereoisomer of A that has all Zdouble bonds.arrow_forward
- Part D. Do the two structures A and B of each pair drawn below represent the same molecule, constitutional isomers, or stereoisomers? If A and B are stereoisomers, further classify them as enantiomers or diastereomers.arrow_forwardThe [α] of pure quinine, an antimalarial drug, is −165. a.Calculate the ee of a solution with the following [α] values: −50, −83, and −120. b. For each ee, calculate the percent of each enantiomer present. c.What is [α] for the enantiomer of quinine? d. If a solution contains 80% quinine and 20% of its enantiomer, what is the ee of the solution? e. What is [α] for the solution described in part (d)?arrow_forwardExplain why compound A has two stereoisomers but compounds B and C exist as single compounds.arrow_forward
- Captopril is a drug used to treat high blood pressure and congestiveheart failure.Draw the enantiomer of captoprilarrow_forwardHow is compound A related to compounds B–E? Choose fromenantiomers, diastereomers, constitutional isomers, or identicalmolecules.arrow_forward(a) Locate the stereogenic centers in the ball-and-stick model of ezetimibe (trade name Zetia), a cholesterol-lowering drug. (b) Label each stereogenic center as R or S.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





