
ORGANIC CHEMISTRY (LL) W/ACCESS
4th Edition
ISBN: 9781119856122
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 54IP
Interpretation Introduction
Interpretation: The difference in reactivity between an ammonium ion and an iminium ion is to be interpreted for the given mechanism.
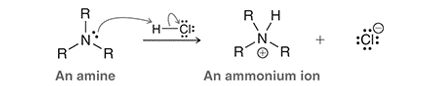
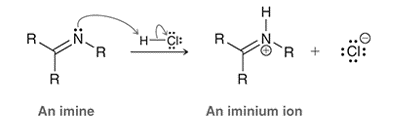
Concept introduction: In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
One acid–base classification defines a base as a substance that acts as a proton (H+H+) acceptor, and is also known as a Bronsted–Lowry base. All bases contain a non‑bonding pair of electrons.
In each of the molecules, identify the atom that behaves only like a Bronsted–Lowry base.
The atom in compound A that behaves only like a Bronsted–Lowry base is
the nitrogen.
the oxygen on the carbonyl.
the oxygen bonded to hydrogen.
the hydrogen bonded to oxygen.
Show how acid derivatives hydrolyze to carboxylic acids under either acidic or basicconditions. Explain why some acid derivatives (amides, for example) require muchstronger conditions for hydrolysis than other derivatives.
Is a carboxylate salt likely to produce acidic or basic solutions in water?
Chapter 6 Solutions
ORGANIC CHEMISTRY (LL) W/ACCESS
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Similar questions
- Carbonyl compounds can be protonated on the carbonyl oxygen. Protonation of the carbonyl oxygen gives a species whose positive charge is delocalized by resonance. Explain why acetic acid (ethanoic acid) is more readily protonated than acetone (propanone).arrow_forwardWhy is the N-H bond of an imide especially acidic? A) The conjugate acid is stabilized by resonance. B) The conjugate base is stabilized by resonance. The conjugate base is stabilized by (c) intramolecular hydrogen bonding. The conjugate base is stabilized by electron- (D donating inductive effect.arrow_forwardArrange the following molecules in increasing order of acidity. Base it only on their structural differences and explain how it is so. 1. HF, CH3CH2CH2OH, CH3CH2COOH 2. Ethyl amine, Ethanol, Propanearrow_forward
- As we shall see in Chapter 19, hydrogens on a carbon adjacent to a carbonyl group are far more acidic than those not adjacent to a carbonyl group. The anion derived from acetone, for example, is more stable than is the anion derived from ethane. Account for the greater stability of the anion from acetone.arrow_forwardDefine central relationship between nucleophilicity and basicity in comparing two nucleophiles?arrow_forwardPut the amines in order of decreasing basicity in (aq) solution: NH3 CH3CH2CH2NH2 (CH3CH2CH2)2NH (CH3CH2CH2)3N I II III IVarrow_forward
- Arrange the following compounds in the increasing order of their acid strength: p-cresol, p-nitrophenol, phenolarrow_forwardArrange the following in increasing order of boiling point. Give reason. Methylamine, trimethylamine, dimethylamine Arrange the following in increasing order of basicity. Give reason. Methylamine, trimethylamine, dimethylamine Arrange the following in increasing order of solubility in H2O. Give reason. Methylamine, trimethylamine, dimethylaminearrow_forwardIn the mid-1930s a substance was isolated from a fungus that is a parasite of ryes and other grasses. This alkaloid, lysergic acid, has been of great interest to chemists because of its strange, dramatic action on the human mind. Many derivatives of lysergic acid are known, some with medicinal applications. Perhaps the best known derivative of lysergic acid is the potent hallucinogen lysergic acid diethylamide (LSD): మగవా జి N-H LSD (CH25N;O) Like other alkaloids, LSD is a weak base, with Kp = 7.6 × 107. What is the pH of a 0.94 M solution of LSD? pH =arrow_forward
- 2. Will a 0.10 molar solution of CH3NH3OCN (methylamine cyanate) produce a basic or acidic solution. Explain quantitatively.arrow_forwardCompounds like amphetamine that contain nitrogen atoms are protonated by the HCl in the gastric juices of the stomach, and the resulting salt is then deprotonated in the basic environment of the intestines to regenerate the neutral form. Write proton transfer reactions for both of these processes. In which form will amphetamine pass through a cell membrane?arrow_forwardAmines as Bases. Explain ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning