
Concept explainers
(a)
Interpretation: The migrating carbon atom and curved arrow for migration are interpreted for the given carbocation rearrangement.
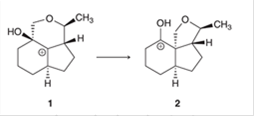
Concept introduction: The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or protons. A carbocation is one of the intermediates that can rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary > Secondary > Primary
(b)
Interpretation: The more stability of carbocation (2) compared to (1) is interpreted for the given carbocation rearrangement.
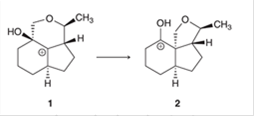
Concept introduction: The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or protons. A carbocation is one of the intermediates that can rearrange to form a more stable carbocation. The stability order of carbocation is:
Tertiary > Secondary > Primary
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY
- #6). Second picture shows how to use arrows correctly.arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction.arrow_forwardDraw the most stable resonance form for the intermediate in the following electrophilic substitution reaction. Ⓒ. CCl₂ • You do not have to consider stereochemistry. . Include all valence lone pairs in your answer. . In cases where there is more than one answer, just draw one. Bo H₂SO4 First stage in synthesis of the insecticide DDT, which is now banned in the US #₂ SAIF CI OH CC13arrow_forward
- 6. a) For each of the following pairs of molecules, identify the stereochemical relationship. Are they enantiomers, diastereomers, constitutional isomers, or the same molecule. CH3 н— сно i) ii) CHO H3C-OH НО HO ОН CI H CI iii) iv) Br H H H CI H. Br CI CI CI b) Explain why carbocation A is formed in step 2, rather than carbocation B (formed by the mechanism shown below). A Barrow_forward2. a) Draw mechanism arrows to show how the reactants are converted to the products. b) Using the pKa values in Table 6.1, determine which side of the equilibrium will be favored. H-CI CląC Cl3C HO, H EH H Earrow_forward6. a. b. C. PRINT the name of the following alkenes. Specify E/Z were necessary.arrow_forward
- 5. a) When compound 5 is treated with methanol it forms a carbocation. Draw the carbocation that would be formed and the best resonance structure of the compound. CH3 5 CH3OH Br b) Below are different conditions that can be used for an SN1 mechanism. In each pair circle the molecule that would provide a faster SN1. If they are equal rates, circle both. a) electrophile b) solvent c) Nucleophile CH3OH CH3OH CH3ONaarrow_forward12. (10 pts) Under the right conditions radical bromination of A produces compound B as shown below (this is termed allylic bromination): hv Br Br2 A B Write a detailed mechanism for the propagation steps of the above radical bromination, showing how the products are formed and that the radical used in the first step is formed in the last step (use curved arrows to show electron flow).arrow_forwardFour carbocations are shown, which can possibly rearrange via hydride shift to a more stable carbocation. H H „CH2 H3C CH3 H3C A D Select the carbocation(s) that will rearrange via hydride shift. B A Darrow_forward
- 5. Chairs and E2 b. a. Draw the line-bond structure of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Draw both chair flips of (1R, 2S,3S)-1-ethyl-2-iodo-3-isopropylcyclohexane. Showing all calculations, determine which is the more stable conformation. C. Which chair flip is able to undergo E2 elimination? Justify your answer. d. Show the product of such an E2 elimination using NaOEt as base.arrow_forwardCan you help?arrow_forward7. Draw a valence-bond (balloon-animal) orbital picture of an ethyl cation. With reference to your drawing, explain how hyperconjugative stabilization works. Also, next to the Lewis structure below, draw one "no-bond" resonance contributing structure that represents hyperconjugative stabilization of the cation. H. 38. H H Harrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
