
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 81SCQ
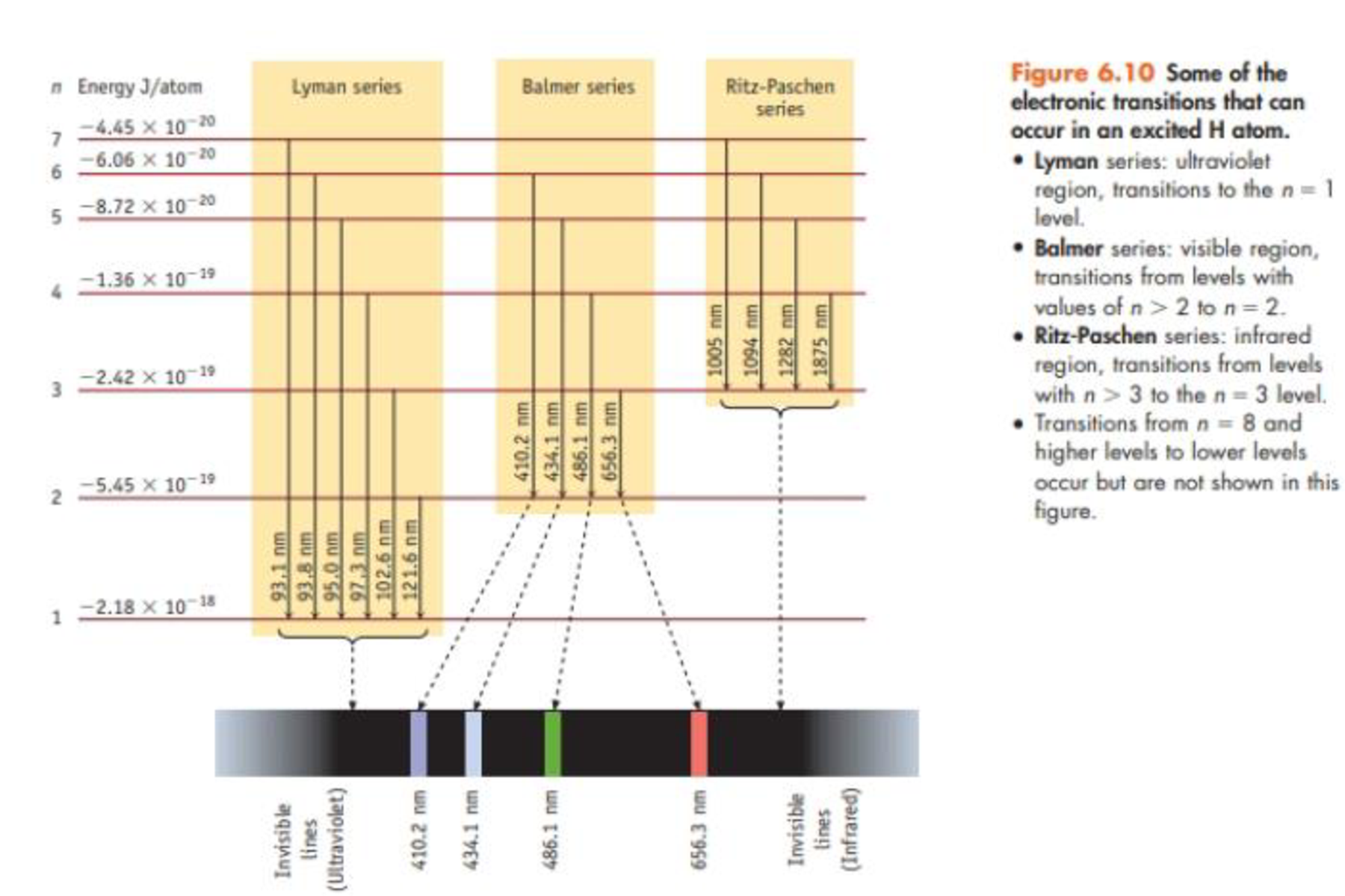
A photon with a wavelength of 93.8 nm strikes a hydrogen atom, and light is emitted by the atom. How many emission lines would be observed? At what wavelengths? Explain briefly (see Figure 6.10).
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 6 Solutions
Chemistry & Chemical Reactivity
Ch. 6.1 - (a) Which color in the visible spectrum has the...Ch. 6.2 - Calculate the energy per mole of photons for the...Ch. 6.3 - Prob. 6.3CYUCh. 6.3 - The Lyman series of spectral lines for the H atom,...Ch. 6.4 - Calculate the wavelength associated with a neutron...Ch. 6.7 - Which has the longer wavelength, visible light or...Ch. 6.7 - Calculate the energy per mole of photons (in...Ch. 6.7 - Prob. 2.1ACPCh. 6.7 - Does the main emission line for SrCl2 have a...Ch. 6.7 - Prob. 2.3ACP
Ch. 6.7 - Helium absorbs light at 587.6 nm. What is the...Ch. 6.7 - Prob. 3.2ACPCh. 6.7 - Prob. 3.3ACPCh. 6.7 - Prob. 3.4ACPCh. 6.7 - Prob. 3.5ACPCh. 6 - Answer the following questions based on Figure...Ch. 6 - Consider the colors of the visible spectrum. (a)...Ch. 6 - Traffic signals are often now made of LEDs...Ch. 6 - Suppose you are standing 225 m from a radio...Ch. 6 - Green light has a wavelength of 5.0 102 nm. What...Ch. 6 - Violet light has wavelength of about 410 nm. What...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - Place the following types of radiation in order of...Ch. 6 - Place the following types of radiation in order of...Ch. 6 - An energy of 3.3 1019 J/atom is required to cause...Ch. 6 - You are an engineer designing a switch that works...Ch. 6 - The most prominent line in the spectrum of mercury...Ch. 6 - The most prominent line in the spectrum of neon is...Ch. 6 - A line in the Balmer series of emission lines of...Ch. 6 - What are the wavelength and frequency of the...Ch. 6 - Consider only transitions involving the n = 1...Ch. 6 - Consider only transitions involving the n = 1...Ch. 6 - The energy emitted when an electron moves from a...Ch. 6 - If energy is absorbed by a hydrogen atom in its...Ch. 6 - Calculate the wavelength and frequency of light...Ch. 6 - Calculate the wavelength and frequency of light...Ch. 6 - An electron moves with a velocity of 2.5 X 108...Ch. 6 - A beam of electrons (m = 9.11 X 1031 kg/electron)...Ch. 6 - Calculate the wavelength, in nanometers,...Ch. 6 - A rifle bullet (mass = 1.50 g) has a velocity of...Ch. 6 - (a) When n = 4, what are the possible values of ?...Ch. 6 - (a) When n = 4, = 2, and m = 1, to what orbital...Ch. 6 - A possible excited state of the H atom has the...Ch. 6 - A possible excited state for the H atom has an...Ch. 6 - How many subshells occur in the electron shell...Ch. 6 - Prob. 32PSCh. 6 - Explain briefly why each of the following is not a...Ch. 6 - Which of the following represent valid sets of...Ch. 6 - What is the maximum number of orbitals that can be...Ch. 6 - What is the maximum number of orbitals that can be...Ch. 6 - Explain briefly why each of the following is not a...Ch. 6 - Explain briefly why each of the following is not a...Ch. 6 - State which of the following orbitals cannot exist...Ch. 6 - State which of the following orbitals cannot exist...Ch. 6 - Write a complete set of quantum numbers (n, , m)...Ch. 6 - Write a complete set of quantum numbers (n, , and...Ch. 6 - A particular orbital has n = 4 and = 2. What must...Ch. 6 - A given orbital has a magnetic quantum number of m...Ch. 6 - Prob. 45PSCh. 6 - Prob. 46PSCh. 6 - Which of the following are applicable when...Ch. 6 - Prob. 48GQCh. 6 - Give the number of nodal surfaces through the...Ch. 6 - What is the maximum number of s orbitals found in...Ch. 6 - Match the values of l shown in the table with...Ch. 6 - Sketch a picture of the 90% boundary surface of an...Ch. 6 - Complete the following table.Ch. 6 - Excited H atoms have many emission lines. One...Ch. 6 - An advertising sign gives off red light and green...Ch. 6 - Radiation in the ultraviolet region of the...Ch. 6 - A cell phone sends signals at about 850 MHz (where...Ch. 6 - Assume your eyes receive a signal consisting of...Ch. 6 - If sufficient energy is absorbed by an atom, an...Ch. 6 - Suppose hydrogen atoms absorb energy so that...Ch. 6 - Rank the following orbitals in the H atom in order...Ch. 6 - How many orbitals correspond to each of the...Ch. 6 - Cobalt-60 is a radioactive isotope used in...Ch. 6 - Exposure to high doses of microwaves can cause...Ch. 6 - When the Sojourner spacecraft landed on Mars in...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - Answer the following questions as a summary quiz...Ch. 6 - Answer the following questions as a summary quiz...Ch. 6 - For an electron in a hydrogen atom, calculate the...Ch. 6 - A solution of KMnO4 absorbs light at 540 nm (page...Ch. 6 - Prob. 71ILCh. 6 - The spectrum shown here is for aspirin. The...Ch. 6 - The infrared spectrum for methanol. CH3OH, is...Ch. 6 - Bohr pictured the electrons of the atom as being...Ch. 6 - Light is given off by a sodium- or...Ch. 6 - Prob. 76SCQCh. 6 - What does wave-particle duality mean? What are its...Ch. 6 - Prob. 79SCQCh. 6 - Suppose you live in a different universe where a...Ch. 6 - A photon with a wavelength of 93.8 nm strikes a...Ch. 6 - Explain why you could or could not measure the...Ch. 6 - Prob. 83SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many electrons in an atom can have the following quantum designation? (a) 1s (b) 4d, m l =0(c) n=5,l=2arrow_forwardThe energy of a photon of visible light emitted by an excited atom is the energy change that takes place within the atom itself.arrow_forwardExplain electron from a quantum mechanical perspective, including a discussion of atomic radii, probabilities, and orbitals.arrow_forward
- The eyes of certain reptiles pass a single visual signal to the brain when the visual receptors are struck by photons of a wavelength of 850 nm. If a total energy of 3.151014 J is required to trip the signal, what is the minimum number of photons that must strike the receptor?arrow_forwardConsider the bright line spectrum of hydrogen shown in Fig. 11.10 . Which line in the spectrum represents photons with the highest energy? With the lowest energy?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
Quantum Mechanics - Part 1: Crash Course Physics #43; Author: CrashCourse;https://www.youtube.com/watch?v=7kb1VT0J3DE;License: Standard YouTube License, CC-BY