
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 93QRT
Aspirin is made from salicylic acid, which has this Lewis structure:
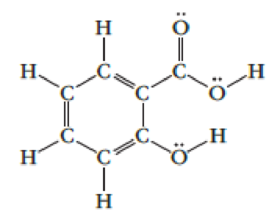
- (a) Which is the longest carbon-carbon bond?
- (b) Which is the strongest carbon-oxygen bond?
- (c) Draw resonance structures for this molecule.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Which of the following pairs represent resonance structures?
Which of the following is the correct Lewis symbol for a neutral atom of F?
Why does the Lewis Structure of Ionic Molecules is written in sucha way that there is no dot or dash between atoms?
Chapter 6 Solutions
Chemistry: The Molecular Science
Ch. 6.2 - Write Lewis structures for (a) NF3, (b) N2H4, and...Ch. 6.3 - Prob. 6.1ECh. 6.3 - Prob. 6.2PSPCh. 6.4 - Prob. 6.2CECh. 6.4 - Write Lewis structures for (a) nitrosyl ion, NO+;...Ch. 6.5 - Prob. 6.4CECh. 6.5 - Prob. 6.5CECh. 6.5 - Prob. 6.4PSPCh. 6.6 - Prob. 6.5PSPCh. 6.6 - Use Equation 6.1 and values from Table 6.2 to...
Ch. 6.6 - Prob. 6.6CECh. 6.7 - Prob. 6.7PSPCh. 6.7 - Prob. 6.7CECh. 6.8 - Prob. 6.8PSPCh. 6.9 - Prob. 6.9PSPCh. 6.9 - Prob. 6.9CECh. 6.10 - Prob. 6.10PSPCh. 6.11 - Prob. 6.10ECh. 6.11 - Prob. 6.11ECh. 6.11 - Prob. 1CECh. 6.11 - Prob. 2CECh. 6.12 - Repeat Problem-Solving Example 6.11, but use N2...Ch. 6.12 - Use MO theory to predict the bond order and the...Ch. 6 - Prob. 1QRTCh. 6 - Prob. 2QRTCh. 6 - Prob. 3QRTCh. 6 - Prob. 4QRTCh. 6 - Prob. 5QRTCh. 6 - Prob. 6QRTCh. 6 - Which of these molecules have an odd number of...Ch. 6 - Prob. 8QRTCh. 6 - Prob. 9QRTCh. 6 - Prob. 10QRTCh. 6 - Prob. 11QRTCh. 6 - Prob. 12QRTCh. 6 - Explain in your own words why the energy of two H...Ch. 6 - Prob. 14QRTCh. 6 - Prob. 15QRTCh. 6 - Prob. 16QRTCh. 6 - Prob. 17QRTCh. 6 - Prob. 18QRTCh. 6 - Prob. 19QRTCh. 6 -
Write Lewis structures for
tetracyanoethene,...Ch. 6 - Prob. 21QRTCh. 6 - Prob. 22QRTCh. 6 - Prob. 23QRTCh. 6 - Prob. 24QRTCh. 6 - Prob. 25QRTCh. 6 - Prob. 26QRTCh. 6 - Prob. 27QRTCh. 6 - Prob. 28QRTCh. 6 - Prob. 29QRTCh. 6 - For each pair of bonds, predict which is the...Ch. 6 - Prob. 31QRTCh. 6 - Prob. 32QRTCh. 6 - Which bond requires more energy to break: the...Ch. 6 -
Estimate ΔrH° for forming 2 mol ammonia from...Ch. 6 - Prob. 35QRTCh. 6 - Light of appropriate wavelength can break chemical...Ch. 6 - Prob. 37QRTCh. 6 - Prob. 38QRTCh. 6 - Prob. 39QRTCh. 6 - Acrolein is the starting material for certain...Ch. 6 - Prob. 41QRTCh. 6 - Prob. 42QRTCh. 6 - Write the correct Lewis structure and assign a...Ch. 6 - Prob. 44QRTCh. 6 - Prob. 45QRTCh. 6 - Two Lewis structures can be written for nitrosyl...Ch. 6 - Prob. 47QRTCh. 6 - Prob. 48QRTCh. 6 - Prob. 49QRTCh. 6 - Prob. 50QRTCh. 6 - Several Lewis structures can be written for...Ch. 6 - Prob. 52QRTCh. 6 - Prob. 53QRTCh. 6 - Prob. 54QRTCh. 6 - Prob. 55QRTCh. 6 - Draw resonance structures for each of these ions:...Ch. 6 - Three known isomers exist of N2CO, with the atoms...Ch. 6 - Write the Lewis structure for (a) BrF5 (b) IF5 (c)...Ch. 6 - Write the Lewis structure for
BrF3
XeF4
Ch. 6 - Prob. 60QRTCh. 6 - Prob. 61QRTCh. 6 - Prob. 62QRTCh. 6 - All carbon-to-carbon bond lengths are identical in...Ch. 6 - Prob. 64QRTCh. 6 - Prob. 65QRTCh. 6 - Prob. 66QRTCh. 6 - Prob. 67QRTCh. 6 - Prob. 68QRTCh. 6 - Prob. 69QRTCh. 6 - Prob. 70QRTCh. 6 - Using just a periodic table (not a table of...Ch. 6 - The CBr bond length in CBr4 is 191 pm; the BrBr...Ch. 6 - Prob. 73QRTCh. 6 -
Acrylonitrile is the building block of the...Ch. 6 - Prob. 75QRTCh. 6 - Write Lewis structures for (a) SCl2 (b) Cl3+ (c)...Ch. 6 - Prob. 77QRTCh. 6 - Prob. 78QRTCh. 6 - A student drew this incorrect Lewis structure for...Ch. 6 - This Lewis structure for SF5+ is drawn...Ch. 6 - Tribromide, Br3, and triiodide, I3, ions are often...Ch. 6 - Explain why nonmetal atoms in Period 3 and beyond...Ch. 6 - Prob. 83QRTCh. 6 - Prob. 84QRTCh. 6 - Prob. 85QRTCh. 6 - Prob. 86QRTCh. 6 - Which of these molecules is least likely to exist:...Ch. 6 - Write the Lewis structure for nitrosyl fluoride,...Ch. 6 - Prob. 91QRTCh. 6 - Methylcyanoacrylate is the active ingredient in...Ch. 6 - Aspirin is made from salicylic acid, which has...Ch. 6 - Prob. 94QRTCh. 6 - Prob. 95QRTCh. 6 - Prob. 96QRTCh. 6 - Prob. 97QRTCh. 6 - Prob. 98QRTCh. 6 - Nitrosyl azide, N4O, is a pale yellow solid first...Ch. 6 - Write the Lewis structures for (a) (Cl2PN)3 (b)...Ch. 6 - Nitrous oxide, N2O, is a linear molecule that has...Ch. 6 - The azide ion, N3, has three resonance hybrid...Ch. 6 - Hydrazoic acid, HN3, has three resonance hybrid...Ch. 6 - Prob. 104QRTCh. 6 - Experimental evidence indicates the existence of...Ch. 6 - Prob. 106QRTCh. 6 - Prob. 107QRTCh. 6 - Pipeline, the active ingredient in black pepper,...Ch. 6 - Sulfur and oxygen form a series of 2 anions...Ch. 6 - Prob. 110QRTCh. 6 - Prob. 111QRTCh. 6 - Prob. 112QRTCh. 6 - Prob. 113QRTCh. 6 - Prob. 114QRTCh. 6 - Prob. 115QRTCh. 6 - Prob. 116QRTCh. 6 - Prob. 117QRTCh. 6 - Prob. 118QRTCh. 6 - Prob. 6.ACPCh. 6 - Prob. 6.BCPCh. 6 - Prob. 6.CCP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forwardWrite all resonance structures of chlorobenzene, C6H5Cl, a molecule with the same cyclic structure as benzene. In all structures, keep the CCl bond as a single bond. Which resonance structures are the most important?arrow_forwardIn which of the following compounds does hydrogen bear a partial negative charge: (a) CH4, (b) NH3, (c) H2O, (d) SiH4 or (e) H2S?arrow_forward
- What are Lewis structures and how are they drawn?arrow_forwardWrite two resonance structures for the formate ion, HCO2‒. (The hydrogen and both oxygen atoms are bonded to the carbon.) What do these structures predict about the carbon-oxygen bond lengths of the formate ion? What do these structures predict about the electrical charge on the oxygen atoms?arrow_forwardThe resonance energy of benzene is?arrow_forward
- For the Lewis electron dot formula for oxygen diiodide, OI2, there are ___ Regions of Electron Density around the O and the shape is ____arrow_forwardIf you include structures in which sulfur has an expanded octet and exclude structures with triple bonds, how many resonance structures can be drawn for sulfur trioxide, SO3?arrow_forward(a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?arrow_forward
- One of the compounds discovered on the surface of Mars in our search for the possibility of life on Mars is the simple organic compound butene (C2H4). Draw a Lewis structure of C2H4. Use ∆EN to determine if it is ionic or covalent, as this will influence how you draw it.arrow_forwardCan the formation of covalent bonds be represented with Lewis symbols? How?arrow_forwardConsider the formate ion, HCO2-, which is the anionformed when formic acid loses an H+ ion. The H and the twoO atoms are bonded to the central C atom. (a) Draw the bestLewis structure(s) for this ion. (b) Are resonance structuresneeded to describe the structure? (c) Would you predict thatthe C¬O bond lengths in the formate ion would be longeror shorter relative to those in CO2?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
Stoichiometry - Chemistry for Massive Creatures: Crash Course Chemistry #6; Author: Crash Course;https://www.youtube.com/watch?v=UL1jmJaUkaQ;License: Standard YouTube License, CC-BY
Bonding (Ionic, Covalent & Metallic) - GCSE Chemistry; Author: Science Shorts;https://www.youtube.com/watch?v=p9MA6Od-zBA;License: Standard YouTube License, CC-BY
General Chemistry 1A. Lecture 12. Two Theories of Bonding.; Author: UCI Open;https://www.youtube.com/watch?v=dLTlL9Z1bh0;License: CC-BY