
Concept explainers
Draw three resonance structures for the hydrogen sulfite ion (), one that obeys the octet rule for the central atom and two that expand the octet of the central atom. Calculate the formal charges on all atoms in each structure and determine which, if airy, of the resonance structures has formal charges that are inconsistent with the elements’ electronegativities.
Interpretation:
The resonance structures for hydrogen sulfite ion should be drawn where one resonance structure must obey octet rule and the other two having expanded octet. Also formal charge of all the resonance structure should be drawn and to identify the resonance structure that has an inconsistent with the electronegativities of the elements.
Concept Introduction:
- Sometimes the chemical bonding of a molecule cannot be represented using a single Lewis structure. In these cases, the chemical bonding are described by delocalization of electrons and is known as resonance.
- In some molecules, there is possibility of more than one Lewis structure where all the structures are equally acceptable. One of the acceptable Lewis structures of these molecules is called resonance structure. All the possible resonance structures are imaginary whereas the resonance hybrid is real.
- These structures will differ only in the arrangement of the electrons not in the relative position of the atomic nuclei.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
- Atoms can be stable even though the number of valence electrons in the atoms in a molecule is more than 8 and is called expanded octet
To identify: the resonance structure of hydrogen sulfite ion
Answer to Problem 10PPA
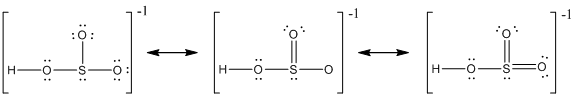
Explanation of Solution
The resonance structure of
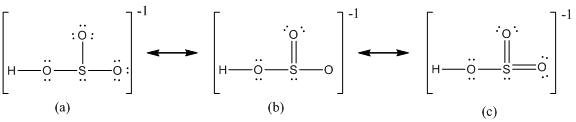
Delocalization of electrons takes place because of the presence of lone pair of electrons in the terminal atoms and the possibility of double bond. The chemical bonding of a molecule cannot be represented using a single Lewis structure and represented by structures (a), (b) and (c). The chemical bonding are described by delocalization of electrons forming three possible resonance structures. In all the resonance structures the position, over whole charge and chemical framework remains intact. Also in these structures only in the arrangement of the electrons differs not the relative position of the atomic nuclei.
Here sulfur shares a double bond with one of the oxygen atom and shares two single bond with remaining two oxygen atoms. Hence in (a), the central sulfur atom is surrounded by 8 electrons therefore it obeys octet rule.
Here sulfur shares a double bond with one of the oxygen atom and shares two single bond with remaining two oxygen atoms. It also have two lone pairs on it. Hence in (b), the central sulfur atom is surrounded by 10 electrons therefore it obeys does not octet rule and is an expanded octet
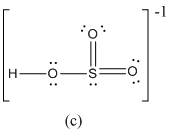
Here sulfur shares two double bond with oxygen atoms and shares a single bond with remaining one oxygen atoms. It also have two lone pairs on it. Hence in (c), the central sulfur atom is surrounded by 12 electrons therefore it obeys does not octet rule and is an expanded octet
Interpretation: the formal charge of atoms of the resonance structure of hydrogen sulfite ion should be determined.
Concept Introduction:
- A formal charge (FC) is the charge assigned to an atom in a molecule, irrespective of relative electronegativity by thinking that electrons in all chemical bonds are shared equally among atoms.
- This method is used to identify the most probable Lewis structures if more than one possibility exists for a compound.
- Formal charge of an atom can be determined by the given formula.
To determine: the formal charge of atoms of the resonance structure of hydrogen sulfite ion.
Answer to Problem 10PPA
Explanation of Solution
For a
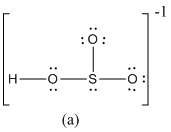
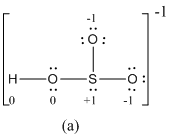
The formal charge of atoms in (a) is calculated.


- Sulfur atom
Substituting,
- Terminal oxygen atoms that has single bond with sulfur atom
Substituting,
- Oxygen atoms that has single bond with sulfur atom and hydrogen
Substituting,
- Hydrogen atom
Substituting,
For b
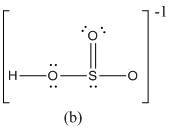
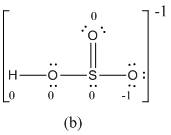
The formal charge of atoms in (b) is calculated.


- Sulfur atom
Substituting,
- Terminal oxygen atom that has single bond with sulfur atom
Substituting,
- Terminal oxygen atom that has double bond with sulfur atom
Substituting,
- Oxygen atoms that has single bond with sulfur atom and hydrogen
Substituting,
- Hydrogen atom
Substituting,
For c
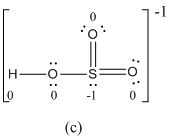
The formal charge of atoms in (c) is calculated.


- Sulfur atom
Substituting,
- Terminal oxygen atoms that has double bond with sulfur atom
Substituting,
Oxygen atoms that has single bond with sulfur atom and hydrogen
Substituting,
Hydrogen atom
Substituting,
- The electronegativity of oxygen is higher than sulfur so usually more electronegative receives negative formal charge. But in the case of resonance structure (c), the less electronegative sulfur atom has -1 formal charge where oxygen gets zero formal charge. Therefore resonance structure (c) has formal charges that are inconsistent with the electronegativities of the elements.
Want to see more full solutions like this?
Chapter 6 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- the formal charges on all the atoms in the following Lewis diagrams. Which one would best represent bonding in the molecule Cl2O ?arrow_forwardDraw resonance structures for each of these ions: NSO− and SNO−. (The atoms are bonded in the order given in each case, that is, S is the central atom in NSO−.) Use formal charges to determine which ion is likely to be more stable. Explain why the two ions cannot be considered resonance structures of each other.arrow_forwardChloromethane has the Lewis structure _______________________________ The carbon atom is sharing 4 electron pairs. In each shared pair the carbon atom “owns” 1 electron. The number of electrons that “belong” to carbon is ___. Carbon, being a Group ___ element would have 4 , outer shell electrons in the unbonded, neutral state. Therefore, the carbon atom in chloromethane has a formal charge of zero.arrow_forward
- The Lewis structure of acetone is Circling the carbonyl carbon, i.e., the carbon atom attached to oxygen, and its octet gives Circling the oxygen atom and its octet gives Thus, atoms share electrons in making bonds, and a pair of electrons may be included in the octet of two different atoms. When computing the formal charge on an atom, the number of electrons that belong to that atom is compared with the number of electrons the atom would have in the unbonded and neutral state. If the two numbers are the same, the formal charge on the atom is zero. In a Lewis structure both electrons in an unshared pair belong to the atom, and one of every pair of shared (bonding) electrons belongs to the atom.arrow_forwardTwo Lewis structures can be written for nitrosyl fluoride, which contains one nitrogen, one oxygen, and one fluorine atom per molecule. Write the two Lewis structures land assign a formal charge to each atom.arrow_forwardA complete Lewis structure must show all nonzero formal charges. Complete each of thefollowing Lewis structures by adding any missing formal charges.arrow_forward
- Sulfur and oxygen form a series of 2 anions including sulfite, SO32, and sulfate, SO42. In addition to these, there are three other more complex anionsdithionite, S2O42, dithionate, S2O62, and tetrathionate, S4O62. Write correct Lewis structures for dithionite, dithionate, and tetrathionate ions.arrow_forwardThe cyclohexane carboxylate anion has a Lewis structure Pushing a pair of unshared electrons away from the negatively charged oxygen atom and, at the same time, pushing a pair of pi electrons toward the other oxygen will generate a second resonance structure. Thus,arrow_forwardWhat is the maximum number of atoms to which a central atom in a molecule can bond and still conform to the octet rule? What is the minimum number?arrow_forward
- Write all possible resonance structures for the following species. Assign a formal charge to each atom. In each case, which resonance structure is the most important? (a) NO2 (nitrogen is central) (b) ClCNarrow_forwardIn the Lewis structure for chloromethane, the chlorine atom is sharing _____ electron pair and “owns” _____ of those electrons. Also, the chlorine atom possesses two electrons from each of _____ unshared pairs. The total number of electrons that belong to chlorine is 7 . Chlorine is a Group ____ element. The formal charge on chlorine in chloromethane is ____.arrow_forwardWrite the Lewis structure and chemical formula of the compound with a molar mass of about 70 g/mol that contains 19.7% nitrogen and 80.3% fluorine by mass, and determine the formal charge of the atoms in this compound.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





