
(a)
Interpretation:
The Lewis electron dot structure for
Concept Introduction:
- Lewis structures are diagrams that represent the
chemical bonding of covalently bonded molecules and coordination compounds. - It is also known as Lewis dot structures which represent the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
- The Lewis structure is based on the concept of the octet rule so that the electrons shared in each atom should have 8 electrons in its outer shell.
Lewis structure for any molecule is drawn by using the following steps,
First the skeletal structure for the given molecule is drawn then the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed such that each atom contains eight electrons in its valence shell.
(a)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for given molecule can be determined by first drawing the skeletal structure. Then, the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
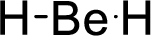
Total number of valence electrons is given below:
Therefore, the Lewis structure is given below:
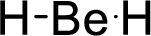
The molecular geometry will be linear because of the presence of two bond pairs around the central atom.
The molecule is a
(b)
Interpretation:
The Lewis electron dot structure for
Concept Introduction:
Refer to (a)
(b)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for given molecule can be determined by first drawing the skeletal structure. Then, the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
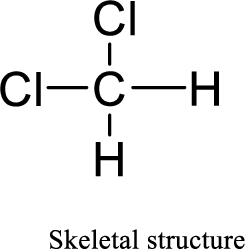
Total number of valence electrons is given below:
Total number of electrons in bonds present is given below:
The twelve electrons remaining will be distributing among chlorine atoms.
Therefore, the Lewis structure is given below:
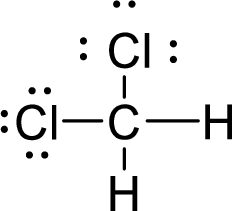
The molecular geometry will be tetrahedral because of the presence of four bond pairs.
The central atom is surrounded with four bond pairs and is a
(c)
Interpretation:
The Lewis electron dot structure for
Concept Introduction:
Refer to (a)
(c)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for given molecule can be determined by first drawing the skeletal structure. Then, the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
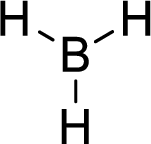
Total number of valence electrons is given below:
Total number of electrons in bonds present is given below:
Therefore, the Lewis structure is given below:
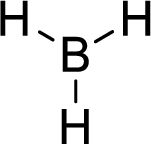
The molecular geometry will be triangular planar because of the presence of three bond pairs.
The central atom is surrounded with three bond pairs and is a
(d)
Interpretation:
The Lewis electron dot structure for
Concept Introduction:
Refer to (a)
(d)
Explanation of Solution
Given molecule is
The Lewis electron dot structure for given molecule can be determined by first drawing the skeletal structure. Then, the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
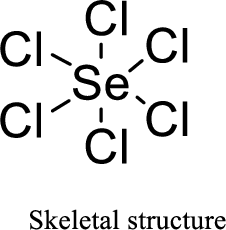
Total number of valence electrons is given below:
Total number of electrons in bonds present is given below:
The thirty-six electrons remaining will be distributing among oxygen atoms.
Therefore, the Lewis structure is given below:
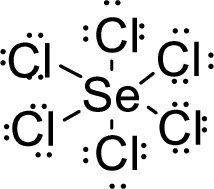
The molecular geometry will be octahedral because of the presence of six bond pairs.
The central atom is surrounded with six bond pairs and is a
(e)
Interpretation:
The Lewis electron dot structure for
Concept Introduction:
Refer to (a)
(e)
Explanation of Solution
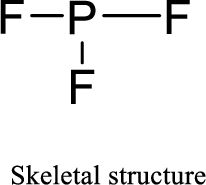
Given molecule is
The Lewis electron dot structure for given molecule can be determined by first drawing the skeletal structure. Then, the total number of valence electrons for all atoms present in the molecule is determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
Total number of valence electrons is given below:
Total number of electrons in bonds present is given below:
The twenty electrons remaining will be distributing among oxygen atoms.
Therefore, the Lewis structure is given below:
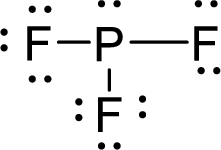
The molecular geometry will be triangular pyramidal because of the presence of three bond pair and one lone pair of electron.
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: The Molecular Science, Hybrid Edition (with OWLv2 24-Months Printed Access Card)
- Ethylamine is an example of an important class of organiccompounds. The molecular formula of ethylamine isCH3CH2NH2. Draw its Lewis structure.arrow_forwardWhat is the molecular shape of CF3-?arrow_forwardHow many lone pairs of electrons are on the central atom of CHCI3 and what is the molecular shape?arrow_forward
- Based on the results of the solubility tests and chemical tests, what is the most probable structure of C7H9N? You may draw the compound using line-bond formula OR Lewis structure.arrow_forward7.40 Why is it impossible for hydrogen to be the central atom in the Lewis structure of a polyatomic molecule?arrow_forwardGiven the bonds C N, C H, C Br, and S O, (a) which atom in each is the more electronegative? (b) which of these bonds is the most polar?arrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning  World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





