
ORGANIC CHEMISTRY 2 YEAR CONNECT ACCES
10th Edition
ISBN: 9781260025309
Author: Carey
Publisher: MCG/CREATE
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 39P
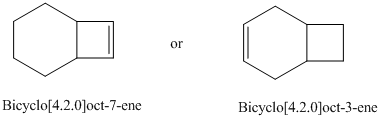
Choose the more stable
Isopropenylcyclopentane or allylcyclopentane
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Kepone, aldrin, and chlordane are synthesized from hexachlorocyclopentadiene and other five-membered-ring compounds. Show how these three pesticides are composed of two five-membered rings.
Give a molecular formula for your product if it contains no oxygens. Give the molecular formulas if your product contains one and two oxygens (some may not be possible).
The molecular formula for if my molecule does not have any oxygens is C6H6, if it has one oxygen it would be C7H6O, if it was two it would be C8H7O2.
Calculate the index of hydrogen deficiency, and therefore the number of rings and/or p- bonds in your unknown for each of the molecular formulas in question 3. Show yourcalculation
Consider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply?
Involves retention of configuration at the site of substitution.
Chapter 7 Solutions
ORGANIC CHEMISTRY 2 YEAR CONNECT ACCES
Ch. 7.1 - Name each of the following using IUPAC...Ch. 7.1 - Prob. 2PCh. 7.2 - How many carbon atoms are sp2-hybridized in the...Ch. 7.3 - Prob. 4PCh. 7.3 - Are cis-2-hexene and trans-3-hexene stereoisomers?...Ch. 7.4 - Prob. 6PCh. 7.4 - Prob. 7PCh. 7.4 - Give the IUPAC name of each of the compounds in...Ch. 7.5 - Arrange the following in order of increasing...Ch. 7.6 - Prob. 10P
Ch. 7.6 - Standard enthalpies of formation are known for all...Ch. 7.6 - Prob. 12PCh. 7.6 - Despite numerous attempts, the alkene...Ch. 7.6 - Write structural formulas for the six isomeric...Ch. 7.7 - Place a double bond in the carbon skeleton shown...Ch. 7.9 - Identify the alkene obtained on dehydration of...Ch. 7.10 - Prob. 17PCh. 7.11 - Prob. 18PCh. 7.12 - Prob. 19PCh. 7.13 - The alkene mixture obtained on dehydration of...Ch. 7.14 - Write the structures of all the alkenes that can...Ch. 7.14 - Write structural formulas for all the alkenes that...Ch. 7.15 - A study of the hydrolysis behavior of...Ch. 7.15 - Use curved arrows to illustrate the electron flow...Ch. 7.15 - Predict the major product of the reaction shown.Ch. 7.16 - Prob. 26PCh. 7.17 - Prob. 27PCh. 7.18 - Prob. 28PCh. 7.19 - Predict the major organic product of each of the...Ch. 7.19 - A standard method for the synthesis of ethers is...Ch. 7 - Write structural formulas for each of the...Ch. 7 - Prob. 32PCh. 7 - Give an IUPAC name for each of the following...Ch. 7 - A hydrocarbon isolated from fish oil and from...Ch. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Choose the more stable alkene in each of the...Ch. 7 - Suggest an explanation for the fact that...Ch. 7 - Prob. 41PCh. 7 - Write structural formulas for all the alkene...Ch. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Predict the major organic product of each of the...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - The rate of the reaction In the first order in...Ch. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - You have available 2,2-dimethylcyclopentanol (A)...Ch. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Acid-catalyzed dehydration of...Ch. 7 - The ratio of elimination to substitution is...Ch. 7 - Prob. 57PCh. 7 - Prob. 58DSPCh. 7 - Prob. 59DSPCh. 7 - Prob. 60DSPCh. 7 - Prob. 61DSPCh. 7 - A Mechanistic Preview of Addition Reactions The...Ch. 7 - Prob. 63DSPCh. 7 - Prob. 64DSPCh. 7 - Prob. 65DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show the process of synthesizing 2-methyl2-cyclohexenone from 2-methyl cyclohexanone. Write down the stages of the monocyclic reaction process in detail.arrow_forwardWhich of the following rings is the most strained? cyclopropene; cyclopropane; cyclobutane; cyclopentane; cyclohexane after finding the most strained compound explain why and compare it to the other answer choices, explaining why they do not work as an answer.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Substitution at a stereocenter gives predominantly a racemic productarrow_forward
- Give a detailed reaction mechanism for the reaction expected to occur when 2-bromo-2-methylpentane is heated with sodium methoxide. Draw clear structural formulas of all relevant species and use curved arrows to represent electron flow. Discuss the stability of the final compoundarrow_forwardExplain why heats of hydrogenation cannot be used to determine the relative stability of 2-methylpent-2-ene and 3-methylpent-1-ene. A, B, and C, each subjected to hydrogenation. The number of rings and π bonds refers to the reactant (A, B, or C) prior to hydrogenation.arrow_forwardGive the IUPAC name of the 1,4-addition product of the reaction between 2-methylpenta-1,3-diene with HCl. No need to specify configuration/isomerism. These are reagents that seek a positive center. What is the IUPAC name of the product between the smallest ring that can form a stable cycloalkyne and hydrogen gas in the presence of palladium in calcium carbonate?arrow_forward
- Write a mechanism that explains how acid-catalyzed dehydration of2,2-dimethylcyclohexanol forms 1,2-dimethylcyclohexene.arrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Involves a carbocation intermediatearrow_forwardDraw and name all stereoisomers of 3-chlorohepta-2,4-diene using the E-Z nomenclature.arrow_forward
- We'll see in the next chapter that the stability of carbocations depends on the number of alkyl substituents attached to the positively charged carbon - the more alkyl substituents there are, the more stable the cation. Which of the two carbocations in each pair is more stable? If they are of equal stablility, specify this.arrow_forward1-pentanol + HBr -> 1-bromopentane Is this substitution reaction reversible? In other words, would one expect to obtain an equilibrium mixture of the alcohol and bromoalkene at the end of the reaction of would one expect this reaction to go to completion? Justify your answer?arrow_forwardThe name of the parent six-membered sulfur-containing heterocycle is thiane. It is numbered beginning at sulfur. Multiple incorporation of sulfur in the ring is indicated by the prefixes di-, tri-, and so on. (a) How many methyl-substituted thianes are there? Which ones are chiral? (b) Write structural formulas for 1,4-dithiane and 1,3,5-trithiane. (c) Which dithiane isomer (1,2-, 1,3-, or 1,4-) is a disulfide?(d) Draw the two most stable conformations of the sulfoxide derived from thiane.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY