
(a)
Interpretation: The reaction mechanism and the configurations of substrate and product for the given reaction should be assigned.
Concept Introduction:
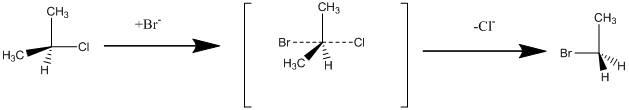
Structure of the substrate plays major role in
Chirality: It refers to a Carbon atom in a molecule that contains four different substituents.
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
R and S nomenclature: it is used to assign the molecule with chiral carbon using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers to the atoms in the decreasing order of their
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Suppose if the hydrogen is placed above the plane then the corresponding isomer is just opposite to the resulting isomer.
(b)
Interpretation: The reaction mechanism and the configurations of substrate and product for the given reaction should be assigned.
Concept Introduction:

Structure of the substrate plays major role in
Chirality: It refers to a Carbon atom in a molecule that contains four different substituents.
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
R and S nomenclature: it is used to assign the molecule with chiral carbon using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers to the atoms in the decreasing order of their atomic mass by placing the hydrogen atom below the plane.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Suppose if the hydrogen is placed above the plane then the corresponding isomer is just opposite to the resulting isomer.
(c)
Interpretation: The reaction mechanism and the configurations of substrate and product for the given reaction should be assigned.
Concept Introduction:

Structure of the substrate plays major role in
Chirality: It refers to a Carbon atom in a molecule that contains four different substituents.
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
R and S nomenclature: it is used to assign the molecule with chiral carbon using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers to the atoms in the decreasing order of their atomic mass by placing the hydrogen atom below the plane.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Suppose if the hydrogen is placed above the plane then the corresponding isomer is just opposite to the resulting isomer.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ORGANIC CHEMISTRY GGC>CUSTOM<-TEXT
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





