
Concept explainers
(a)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form a different compound than the reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In the nucleophilic elimination step, the more electronegative atom bears full or partial negative charge. This is an electron rich atom, and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from the electron rich site to the electron poor site. The second curved arrow is drawn to represent the breaking of the bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrows is drawn as:
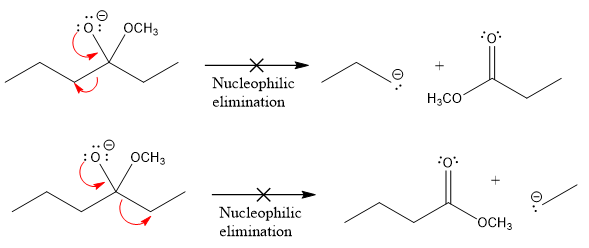
Explanation of Solution
Product for the given nucleophilic addition step is:
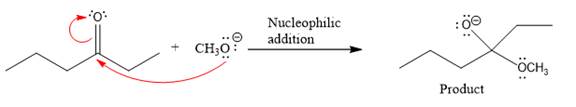
In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is an electron rich site, and the carbon bonded to it is an electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
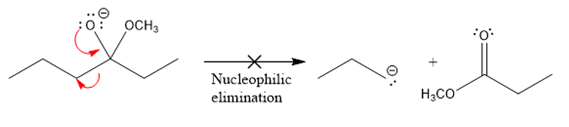
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents that this nucleophilic elimination is unfeasible as
In the second nucleophilic elimination step, the oxygen atom with negative charge is the electron rich site, and the carbon bonded to it is the electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
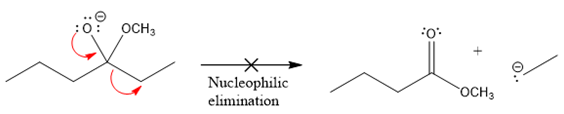
The first curve arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents that this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(b)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form a different compound than the reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In the nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from the electron rich site to the electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
The product formed after the elimination of the leaving group is not the same as the reactant. Product formed in the nucleophilic elimination step with an appropriate curved arrow is drawn as:
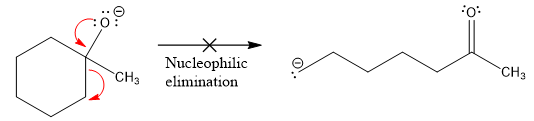
Explanation of Solution
Product for the given nucleophilic addition step is:
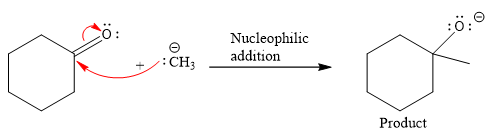
In the nucleophilic elimination step, the oxygen atom with negative charge is an electron rich site, and the carbon bonded to it is an electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Product formed in the elimination step is different from the reactant in the given nucleophilic addition step.
(c)
Interpretation:
The product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
The products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
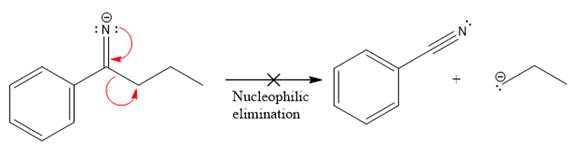
Explanation of Solution
Product for the given nucleophilic addition step is:
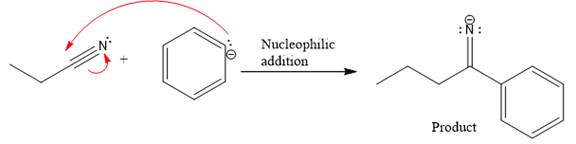
In the nucleophilic elimination step, the nitrogen atom with negative charge is electron rich site, and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged nitrogen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Product formed in the elimination step is different from the reactant in the given nucleophilic addition step.
(d)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not the same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
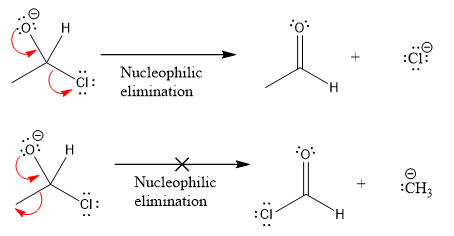
Explanation of Solution
Product for the given nucleophilic addition step is:
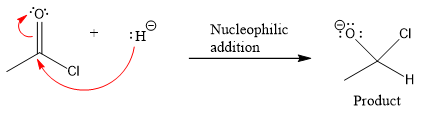
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site, and the chlorine atom is a good leaving group. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
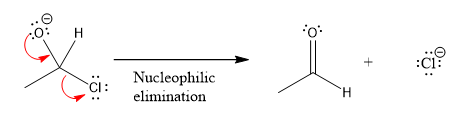
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step.
In the second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
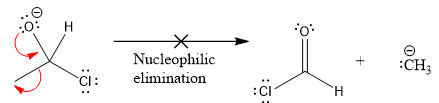
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(e)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow is drawn from the lone pair of electron rich atom points to the bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
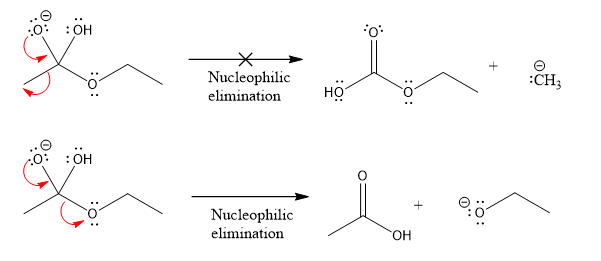
Explanation of Solution
Product for the given nucleophilic addition step is:
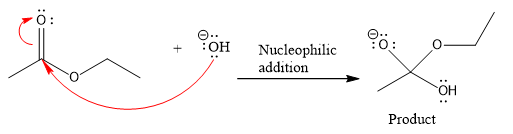
In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site, and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:
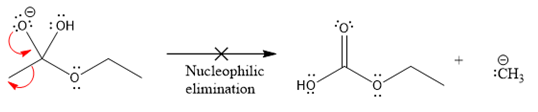
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
In the second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and
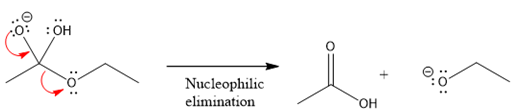
The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step.
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
(f)
Interpretation:
Whether the product of the given step can eliminate a leaving group to form different compound than reactant is to be predicted. The product for the given nucleophilic elimination step with appropriate curved arrows is to be drawn.
Concept introduction:
In nucleophilic elimination step, the more electronegative atom bears full negative charge or partial negative charge. This is the electron rich atom and the less electronegative atom is relatively electron poor. The curved arrow drawn from the lone pair of electron rich atom points to bonding region between the more electronegative atom and less electronegative atom representing the electron flow from electron rich site to electron poor site. The second curved arrow is drawn to represent the breaking of bond between the less electronegative atom and leaving group to avoid exceeding an octet on the less electronegative atom.
Answer to Problem 7.27P
Products formed after the elimination of the leaving group are not same as the reactant. Product formed in the nucleophilic elimination step with appropriate curved arrow is drawn as:
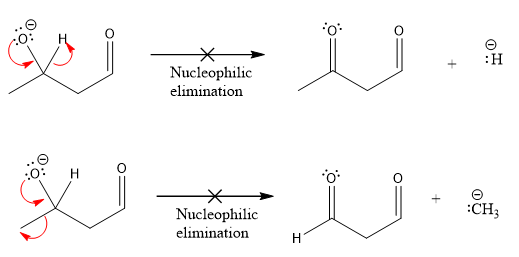
Explanation of Solution
Product for the given nucleophilic addition step is:

In the given product, there are two possible groups that can leave to form two different products.
In the first nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
The second nucleophilic elimination step, the oxygen atom with negative charge is electron rich site and the carbon bonded to it is electron poor site. The curved arrow mechanism for this given nucleophilic elimination step forming the respective product is:

The first curved arrow is drawn from the lone pair of negatively charged oxygen to the mid of
The respective product formed is different from the reactant in the given nucleophilic addition step. The X sign on the arrow represents this nucleophilic elimination is unfeasible as
Products formed in the elimination steps are different from the reactant in the given nucleophilic addition step.
Want to see more full solutions like this?
Chapter 7 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Highlight the electrophilic carbon in red, and highlight the leaving group in blue. Highlight the atom in the nucleophile that will attack the electrophilic center in green. Only atoms need to be highlighted and not the lone pairs or formal chargesarrow_forwardAdd curved arrows to draw step four of the mechanism. Modify the given drawing of the product as needed to show the intermediate that is formed in the step.arrow_forwardUse a scrap piece of paper to draw the curved arrow mechanism for the reaction below. Use your mechanism to determine the major organic product and draw it in the space provided.arrow_forward
- The same reaction with chlorine is not much regioselective in the above. Explain the decreased regioselectivity of the following chlorination reaction by comparing the bromination reaction in the above (draw the mechanism, transition state, energy diagram, etc.). ‘Markovnikov’s or anti-Markovnikov’s rule’ is not a correct answer.arrow_forwardA pair of diastereomers is each individually mixed with a strong base, and for both, an E2 reaction occurs.Provide the missing curved arrow notation, and draw the correct geometric isomer that is formed for both of the E2 mechanisms. There should be TWO diastereomer drawings. (See image...1st box with curved arrows are correct)arrow_forwardDraw all the steps of this mechanism with arrows.arrow_forward
- Find the error in the given answer? Question: For the given starting material, draw the product of the following reaction series.arrow_forwardIdentify the stronger nucleophile in the following pair of anions. HS− or F− in a polar protic solventarrow_forwardIdentify the stronger nucleophile in the following pair of anions. Br− or Cl− in a polar protic solventarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
