
Concept explainers
(a)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
In a bimolecular nucleophilic substitution step (
Answer to Problem 7.37P
For the given elementary step of a
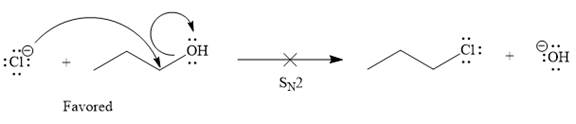
Explanation of Solution
The given elementary step is
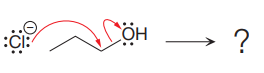
The given elementary step is for an
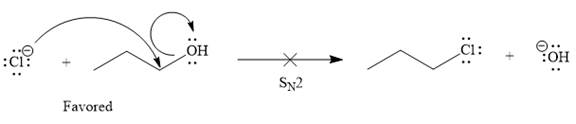
According to the charge stability principle, the negative charge on chlorine is more stable than that on the oxygen atom. Also, the bond energy for the reactant side is higher as compared to the product side. For a reaction or elementary step involving only uncharged species, the side having greater bond energies is favored. Thus, for the above mentioned reaction, the reactant side is favored over the product side.
For a reaction or elementary step involving only uncharged species, the side having greater bond energies is favored.
(b)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
In a bimolecular elimination step (
Answer to Problem 7.37P
For the given elementary step of an
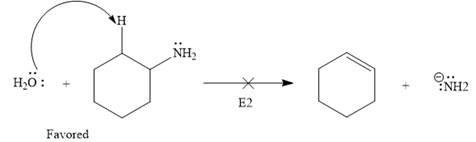
Explanation of Solution
The given elementary step is
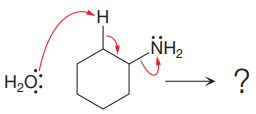
The given elementary step is for an
The products for the above reaction, if we assume that the reaction is complete, are as follows:
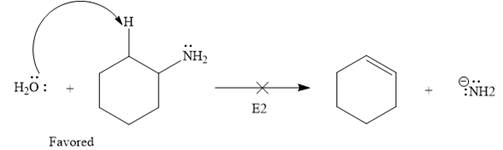
The product side of the above reaction has two charged species, and a sigma bond is replaced by a pi bond. According to the charge stability principle and total bond energy, the reaction favors the reactant side as the bond energy for the reactant side is more than that of the product.
For a reaction or elementary step involving only uncharged species, the side having greater bond energies and greater charge stability is favored.
(c)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
In a nucleophilic addition step, the nucleophile adds to the polar pi bond. Thus, the nucleophile forms a bond with the less electronegative atom and the pi bond breaks, becoming a lone pair on the more electronegative atom.
A sigma bond between the substrate and the nucleophile forms, whereas the polar pi bond breaks.
Driving force is responsible for an elementary step to go to completion. The driving force for a reaction is the extent to which the reaction favors products over reactants, and that tendency increases with increasing stability of the products relative to the reactants. Charge stability and total bond energy are the two major factors that contribute to a reaction’s driving force. Bond energy refers to the amount of energy needed to break a bond. For a reaction or elementary step involving both ions and uncharged molecules, the side that is favored exhibits greater charge stability. For a reaction or elementary step involving only uncharged species, the side that is favored has greater bond energies.
Answer to Problem 7.37P
For the given elementary step of a nucleophilic addition, the product side is favored over the reactant side.
Explanation of Solution
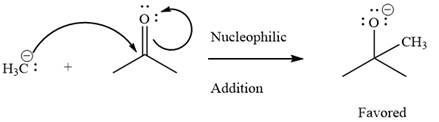
The given elementary step is
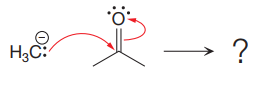
The given elementary step is for a nucleophilic addition.
In the above reaction, the negatively charged carbon serves as a nucleophile and attacks the polar pi bond. The product for the reaction is a charged species with a negative charge on the oxygen atom.
According to the charge stability principle, the negative charge is more stable on the oxygen atom than it is on the carbon atom. So, the reaction is favored in the forward direction. Hence, for the given reaction, the product side is favored over the reactant side.

For a reaction or elementary step involving only uncharged species, the side having greater bond energies and greater charge stability is favored.
(d)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
The electrophilic addition step occurs when a species containing a nonpolar pi bond approaches a strongly electron-deficient species, that is, an electrophile, and a bond is formed between an atom of the pi bond and the electrophile.
Driving force is responsible for an elementary step to go to completion. The driving force for a reaction is the extent to which the reaction favors products over reactants, and that tendency increases with increasing stability of the products relative to the reactants. Charge stability and total bond energy are the two major factors that contribute to a reaction’s driving force. Bond energy refers to the amount of energy needed to break a bond. For a reaction or elementary step involving both ions and uncharged molecules, the side that is favored exhibits greater charge stability. For a reaction or elementary step involving only uncharged species, the side that is favored has greater bond energies.
Answer to Problem 7.37P
For the given elementary step of electrophilic addition, the product side is favored over the reactant side.
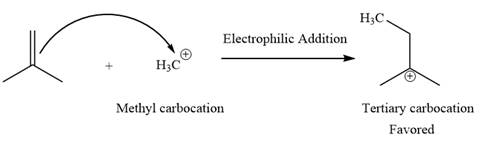
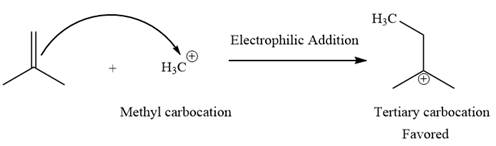
Explanation of Solution
The given elementary step is
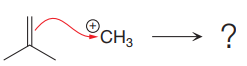
The given elementary step is for electrophilic addition.
In the above reaction, the nonpolar pi bond attacks the positively charged methyl cation. The product for this reaction is a tertiary carbocation. The methyl carbocation on the reactant side is less stable than the tertiary carbocation on the product side. Therefore, the product side is favored over the reactant side for the above reaction.
For a reaction or elementary step involving only uncharged species, the side having greater bond energies and greater charge stability is favored.
(e)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
The electrophilic elimination step is the reverse of the electrophilic addition step.
In this step, an electrophile is eliminated from the carbocation which generates a stable, uncharged species. A pi bond is formed during the process.
Driving force is responsible for an elementary step to go to completion. The driving force for a reaction is the extent to which the reaction favors products over reactants, and that tendency increases with increasing stability of the products relative to the reactants. Charge stability and total bond energy are the two major factors that contribute to a reaction’s driving force. Bond energy refers to the amount of energy needed to break a bond. For a reaction or elementary step involving both ions and uncharged molecules, the side that is favored exhibits greater charge stability. For a reaction or elementary step involving only uncharged species, the side that is favored has greater bond energies.
Answer to Problem 7.37P
For the given elementary step of an electrophilic elimination reaction, the product side is favored over the reactant side.

Explanation of Solution
The given elementary step is
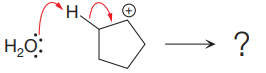
The given elementary step is for electrophilic elimination.
In the above reaction, the electrophile, that is, the proton is removed or eliminated, and a new pi bond is formed between the two carbon atoms. The product for this step is shown as follows:

The carbocation on the reactant side has an incomplete octet, whereas all atoms in the products have their octets complete. Therefore, the product side is favored over the reactant side.
For a reaction or elementary step involving only uncharged species, the side having greater bond energies and greater charge stability is favored.
(f)
Interpretation:
For the given elementary step, it is to be determined whether the reactant or product side is favored.
Concept introduction:
In the co-ordination step, the electrons from an electron rich site flow to an electron-poor site.
In this step, a new bond known as a co-ordinate bond is formed between the electron-rich and electron-poor atoms.
Driving force is responsible for an elementary step to go to completion. The driving force for a reaction is the extent to which the reaction favors products over reactants, and that tendency increases with increasing stability of the products relative to the reactants. Charge stability and total bond energy are the two major factors that contribute to a reaction’s driving force. Bond energy refers to the amount of energy needed to break a bond. For a reaction or elementary step involving both ions and uncharged molecules, the side that is favored exhibits greater charge stability. For a reaction or elementary step involving only uncharged species, the side that is favored has greater bond energies.
Answer to Problem 7.37P
For the given co-ordination step, the product side is favored over the reactant side.
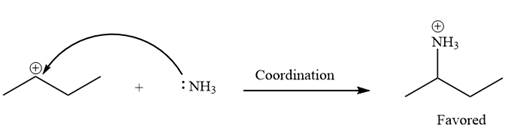
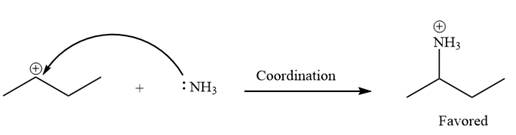
Explanation of Solution
The given elementary step is
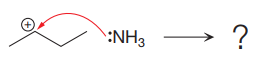
The given elementary step is for the co-ordination reaction.
In the above reaction, the lone pairs of electrons on the nitrogen atom forms a co-ordinate bond with the carbon atom bearing the positive charge. The product for this reaction is shown as below:

The reactant side has a carbocation with an incomplete octet. In the product side, the octets for all the species are complete. Thus, the carbocation on the reactant side is less stable that the positively charged nitrogen atom on the product side. Therefore, the product side is favored over the reactant side.
For a reaction or elementary step involving only uncharged species, the side having greater bond energies and greater charge stability is favored.
Want to see more full solutions like this?
Chapter 7 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- Which rxn would be fastest? Which would be slowest?arrow_forwardDraw the curved arrow notation and products for the each elementary step described by the sequence shown here.Note that the products of the first step should be used as the reactants in the second step, and remember to click on each box to see step-specific instructions.arrow_forwardDrawing the Product(s) of a Mechanistic Step. For the reactions that have TWO starting materials, indicate/label which species is the nucleophile and which isthe electrophile. Then, by following the curved electron-pushing arrows, draw the product(s) of the following reaction steps. Finally, identify and label what type of elementary step is represented for each mechanism.arrow_forward
- OCHEM help... What is the major product of the following reaction sequence? (See attached image)arrow_forwardThe reaction shown here is a halosulfonation, which is a useful variation of the sulfonation reaction. Draw the complete mechanism for this reaction.arrow_forwardPlease draw a full mechanism of the reaction. Please do not copy from previously answered question.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
