
(a)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
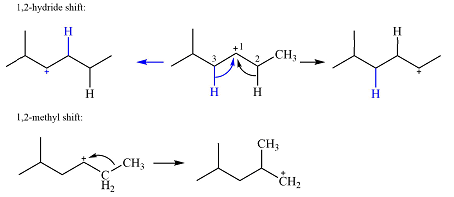
Explanation of Solution
The structure of the carbocation is
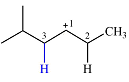
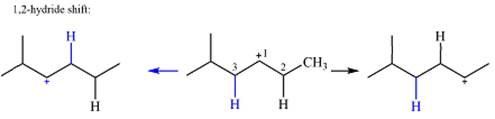
The charge is on the carbon numbered 1. There are two hydrogen atoms on the adjacent carbons C2 and C3. These are the ones that can shift to C1 in the two possible
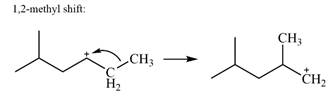
There is only one methyl group on the carbon adjacent to C1, attached to C2. Shifting of this methyl to C1 results in shifting of the charge to C2. Therefore, the
A hydride (
(b)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
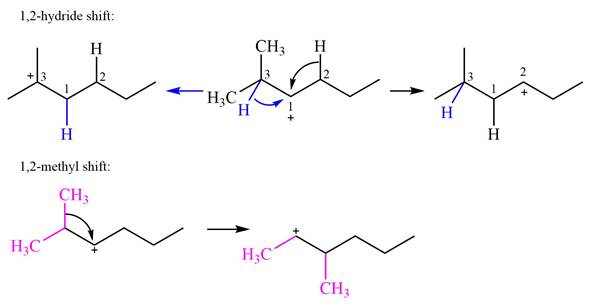
Explanation of Solution
The structure of the given carbocation is
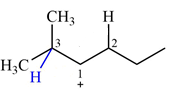
There are two hydrogen atoms on adjacent carbons C2 and C3 that can shift to the positively charged carbon C1 in two possible
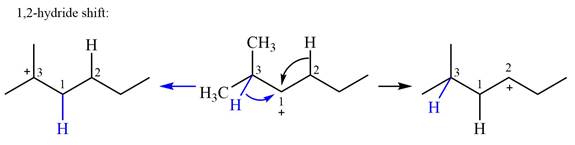
There are two methyl groups attached to a carbon adjacent to C1. Both are on the same carbon C3, therefore, shifting of either one will give the same product.
Therefore, the
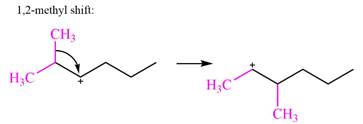
A hydride (
(c)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
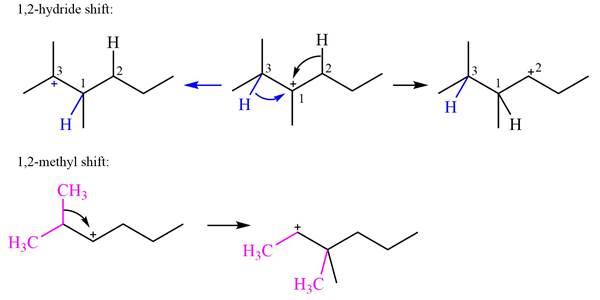
Explanation of Solution
The structure of the given carbocation is
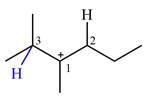
There are two hydrogen atoms on adjacent carbons C2 and C3 that can shift to the positively charged carbon C1 in two possible
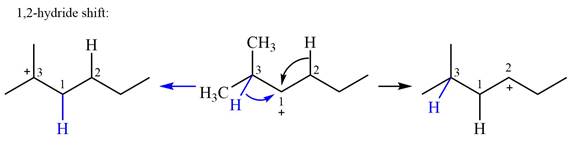
There are two methyl groups attached to a carbon adjacent to C1. Both are on the same carbon C3; therefore, shifting of either one will give the same product.
Therefore, the
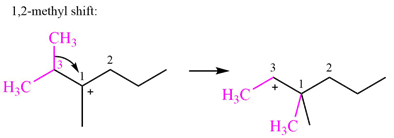
A hydride (
(d)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
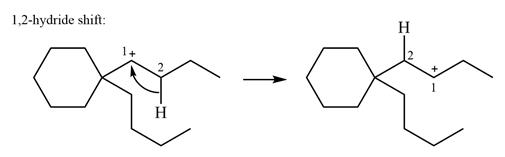
As there are no methyl groups on the carbon adjacent to the charge bearing carbon C1, a
Explanation of Solution
The structure of the given carbocation is
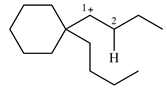
There is only one hydrogen atom on an adjacent carbon, C2, that can shift to the positively charged carbon C1 in a possible
Therefore, the
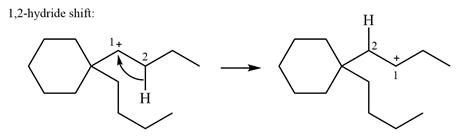
There are no methyl groups attached to the carbon adjacent to C1. Therefore, a
A hydride (
(e)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
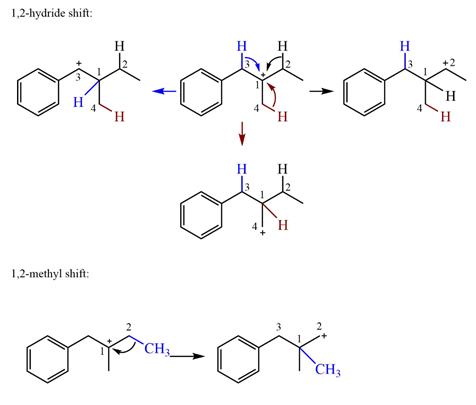
Explanation of Solution
The structure of the given carbocation is
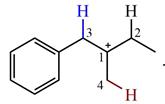
There are three hydrogen atoms on carbon atoms adjacent to the charge carrying carbon. They are on C2, C3, and C4.
Shifting of the hydride on C2 results in the charge shifting to C2, as shown in the product on the right.
Shifting of the hydride on C3 results in the charge shifting to C3, as shown in the product on the left.
Shifting of the hydride on C4 results in the charge shifting to C4, as shown in the product below the given carbocation.
Therefore, the
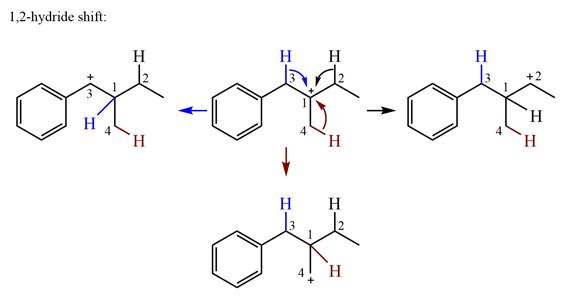
There is one methyl group attached to the carbon adjacent to C1. Shifting of the methyl group on C2 to C1 results in C1 becoming a tertiary carbon and the charge shifting to C2.
Therefore, the possible
A hydride (
(f)
Interpretation:
The curved arrow notations showing all possible
Concept introduction:
Curved arrows are used to represent the movement of electrons in a reaction mechanism. The arrow starts on an electron-rich atom or an electron-rich region such as a pi bond. It ends on an electron poor atom when the movement results in the formation of a new sigma bond. If the result is the formation of a pi bond, the arrow ends in the region between the two atoms that form the bond.
A carbocation is a positively charged carbon atom that is electron-poor, two electrons short of an octet. It is unstable because it is a charged species.
A
A
The numbering in the shift label simply signifies that a hydride or a methyl group migrates from one carbon to an adjacent one. The numbering is not related to the root chain atom numbering.
Answer to Problem 7.36P
The possible
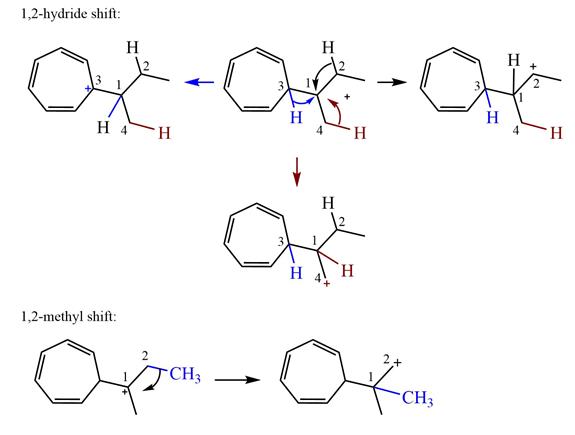
Explanation of Solution
The structure of the given carbocation is
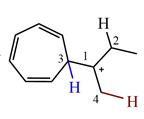
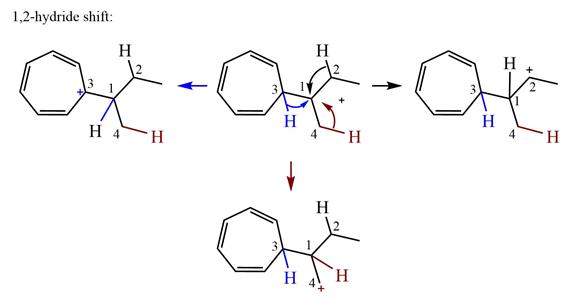
There are three hydrogen atoms on carbon atoms adjacent to the charge carrying carbon. They are on C2, C3, and C4.
Shifting of the hydride on C2 results in the charge shifting to C2, as shown in the product on the right.
Shifting of the hydride on C3 results in the charge shifting to C3, as shown in the product on the left.
Shifting of the hydride on C4 results in the charge shifting to C4, as shown in the product below the given carbocation.
Therefore, the
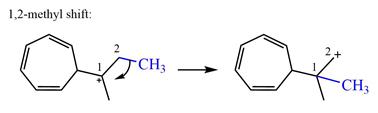
There is one methyl group attached to a carbon adjacent to C1. Shifting of the methyl group on C2 to C1 results in C1 becoming a tertiary carbon and the charge shifting to C2.
Therefore, the possible
A hydride (
Want to see more full solutions like this?
Chapter 7 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- In (e), please note that the heterocyclic oxygen atoms contains two lone pairs that could contribute to the resonance stabilization of any proposed intermediate.arrow_forwardFor each of the following, there are two possible radical addition steps. In each case, draw the curved arrow notation and product for both radical additions and predict the more stable product.arrow_forwardDetermine whether a 1,2-hydride shift is favorable for the following carbocation. If no rearrangement is expected to occur, simply draw the starting carbocation in the second box. If a rearrangement is favorable, use the curved arrow notation to show the hydride shift and draw the structure of the expected carbocation in the second box.arrow_forward
- I am supposed to draw the molecule with the carbocation rearranged to where it is more stable. I was going to to a hydride shift and move it to the carbon that is highlighted in red. The red carbon means it wont let me place it there for some reason. Is that not the most stable position to put it in or is there a better spot?arrow_forwardAdd the curved arrow notation for this elementary step.Classify the step as either homolysis, radical coupling, SH2, or radical addition.arrow_forwardFor the reaction below provide all possible product(s) and circle the major product.arrow_forward
- Draw curved arrows to represent the flow of electron during the reaction for each of thefollowing reactions.arrow_forwardShow the curved arrow formalism in order to arrive in the major final productarrow_forwardNow point out (Circle) the MAJOR CONTRIBUTOR(S) in you need consider all the resonances including the original onesarrow_forward
- Synthesis problem: does this do an expansion? Please provide an explanation! Especially of all regaents.arrow_forwardDraw a mechanism for the reaction of methylamine with formic acid. In the box to the left, draw any necessary curved arrows. Show the products of the reaction in the box to the right. Include any nonzero formal charges and all lone pairs of electrons. Finally, check the box to indicate which side of the reaction is favored at equilibrium.arrow_forwardSolve correctly please, with some explanation also. Draw the product(s) for the following radical reaction where one bond breaks and another bond is formed. The curved arrows indicate the movement of an unpaired electron.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
