
Concept explainers
(a)
Interpretation:
The expected products for the given incomplete reaction has to be drawn.
(b)
Interpretation:
The expected products for the given incomplete reaction has to be drawn.
Concept Introduction:
Hydroboration-oxidation:
Alkyne undergoes hydroboration-oxidation with alkaline peroxide that gives an enol which is in equilibrium with keto-enol tautomerism with octanal.

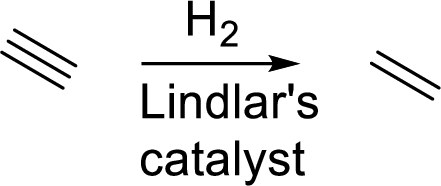
(c)
Interpretation:
The expected products for the given incomplete reaction has to be drawn.
Concept Introduction:
Catalytic hydrogenation: It is the addition of molecular hydrogen across triple bond in presence of Lindlar’s catalyst. This catalyst stops hydrogenation to
Trending nowThis is a popular solution!

Chapter 7 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Starting from bromoethane, the formation of which of the following compound requires more than one step of reaction? 2 (a) Methoxyethane (b) Ethanol (c) Ethanoic acid (d) Ethenearrow_forwardShow how you would use simple chemical tests to distinguish between the following pairs of compounds. In each case, describe what you would do and what you would observe. (a) butan-1-ol and butan-2-ol (b) butan-2-ol and 2-methylbutan-2-olarrow_forwardWhich of the following statements about 3-iodo-2-methylprop-1-ene is/are true? (i) It has an E isomer and a Z isomer. (ii) It can be converted to an alkyl lithium compound in one step by reacting with lithium metal. (iii) It would react with the following reagent to give 2-methylhept-1-ene as the product: CuLiarrow_forward
- 11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forwardPredict the products of the sulfuric acid-catalyzed dehydration of the following alcohols. When more than one product is expected, label the major and minor products. (a) 2-methylbutan-2-ol (b) pentan-1-olarrow_forwardShow how you would use simple chemical tests to distinguish between the following pairs of compounds. In each case,describe what you would do and what you would observe.(a) butan-1-ol and butan-2-olarrow_forward
- Which reactions will produce the desired product in good yield? You may assume thataluminum chloride is added as a catalyst in each case. For the reactions that will not givea good yield of the desired product, predict the major products.Reagents Desired Product(a) benzene + n-butyl bromide n-butylbenzenearrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanoarrow_forwardWhich reactions will produce the desired product in good yield? You may assume that aluminum chloride is added as a catalyst in each case. For the reactions that will not give a good yield of the desired product, predict the major products. Reagents Desired Product(a) benzene + n-butyl bromide n-butylbenzene(b) ethylbenzene + tert-butyl chloride p-ethyl-tert-butylbenzene(c) bromobenzene + ethyl chloride p-bromoethylbenzene(d) benzamide (PhCONH2) + CH3CH2Cl p-ethylbenzamide(e) toluene + HNO3, H2SO4, heat 2,4,6-trinitrotoluene (TNT)arrow_forward
- Predict the major products formed when benzene reacts (just once) with the following reagents.(a) tert-butyl bromide, AlCl3arrow_forwardGrignard reagent is a versatile tool in synthetic organic chemistry. Using bromocyclopentane as a starting material, show how a Grignard reagent, X, is synthesized. Reaction of X with water produces compound Y while treatment in carbon dioxide followed by hydrolysis forms compound Z. 3-methyl-2butanone reacts with X and hydrolyses to yield compound AA. Draw the structural formulae of compounds Y, Z and AA and write the chemical equations respectively.arrow_forwardWhich reactions will produce the desired product in good yield? You may assume thataluminum chloride is added as a catalyst in each case. For the reactions that will not givea good yield of the desired product, predict the major products.Reagents Desired Product(a) benzene + n-butyl bromide n-butylbenzene(b) ethylbenzene + tert-butyl chloride p-ethyl-tert-butylbenzenearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





