
Water at 20 psia and 50°F enters a mixing chamber at a rate of 300 lbm/min where it is mixed steadily with steam entering at 20 psia and 240°F. The mixture leaves the chamber at 20 psia and 130°F, and heat is lost to the surrounding air at 70°F at a rate of 180 Btu/min. Neglecting the changes in kinetic and potential energies, determine the rate of entropy generation during this process.
FIGURE P7–140E
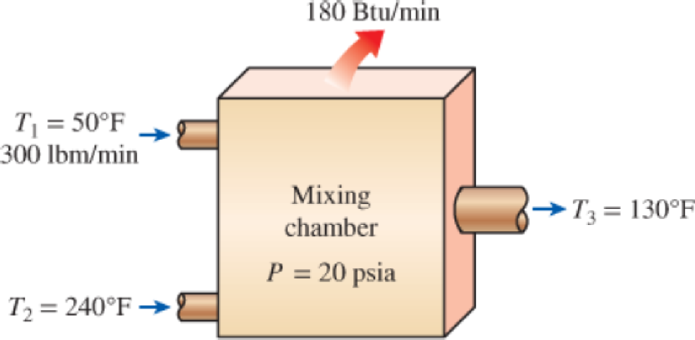
The rate of entropy generation during the process.
Answer to Problem 139P
The rate of entropy generation during the process is
Explanation of Solution
Write the expression for the energy balance equation for closed system.
Here, rate of net energy transfer in to the control volume is
The rate of change in internal energy of the system is zero at steady state,
Write the expression for the mass balance of the system.
Here, inlet mass flow rate is
Write the expression for the entropy balance during the process.
Here, rate of net input entropy is
Conclusion:
Substitute 0 for
Here, mass flow rate at inlet 1 is
Substitute
Substitute
Refer to Table A-4E, “Saturated water—Temperature table”, obtain the below properties at the pressure of
Here, fluid entropy is
Refer to Table A-4E, “Saturated water—Temperature table”, obtain the below properties at the pressure of
Here, entropy at inlet 2 is
Refer to Table A-4E, “Saturated water—Temperature table”, obtain the below properties at the pressure of
Here, entropy at exit 3 is
Substitute
Substitute
Substitute
Substitute
Thus, the rate of entropy generation during the process is
Want to see more full solutions like this?
Chapter 7 Solutions
Thermodynamics: An Engineering Approach
- Refrigerant-134a enters a compressor as a saturated vapor at 160 kPa at a rate of 0.03 m3 /s and leaves at 800 kPa. The power input to the compressor is 10 kW. If the surroundings at 20°C experience an entropy increase of 0.008 kW/K, determine the rate of entropy generation.arrow_forwardSteam enters an adiabatic turbine steadily at 7 MPa, 500°C, and 45 m/s and leaves at 100 kPa and 75 m/s. If the power output of the turbine is 5 MW and the isentropic efficiency is 77 percent, determine the temperature at the turbine exit.arrow_forwardAn adiabatic diffuser at the inlet of a jet engine increases the pressure of the air that enters the diffuser at 11 psia and 30°F to 20 psia. What will the air velocity at the diffuser exit be if the diffuser isentropic efficiency, defined as the ratio of the actual kinetic energy change to the isentropic kinetic energy change, is 82 percent and the diffuser inlet velocity is 1200 ft/s?arrow_forward
- A piston is inserted into a cylinder causing the pressure in the air to change from 50 to 4000 kPa while the temperature remains constant at 27°C. To accomplish this, heat transfer must occur. Determine the entropy change.arrow_forwardWater at 20 psia and 500F enters a mixing chamber at a rate of 7 lbm/s where it is mixed steadily with steam entering at 20 psia and 2800F. The mixture leaves the chamber at a rate of 500 Ibm/min at 20 psia and 1600F, and heat is lost to the surrounding air at 700F at a rate of 200 Btu/min. Neglecting the changes in kinetic and potential energies, determine the rate of entropy generation during this process.arrow_forwardSteam enters an adiabatic turbine steadily at 7 MPa, 500°C, and 45 m/s and leaves at 100 kPa and 75 m/s. If the power output of the turbine is 5 MW and the isentropic efficiency is 77 percent, determine the mass flow rate of steam through the turbine.arrow_forward
- In a production facility, 1.2-in-thick, 2-ft × 2-ft square brass plates (ρ = 532.5 lbm/ft3 and cp = 0.091 Btu/lbm·°F) that are initially at a uniform temperature of 75°F are heated by passing them through an oven at 1300°F at a rate of 450 per minute. If the plates remain in the oven until their average temperature rises to 1000°F, determine the rate of entropy generation associated with this heat transfer process.arrow_forwardSteam enters an adiabatic turbine at 5 MPa and 500ºC with a 50 m/s velocity and exits from the turbine at 100 kPa and 75 m/s velocity. The power output of the turbine is 5 MW and the isentropic efficiency is 80%. Determine,a) The mass flow rate of the steam passed through the turbine.b) The temperature at the turbine exit.c) The rate of entropy generation during this processarrow_forwardIs it possible for the entropy change of a closed system to be zero during an irreversible process? Explain.arrow_forward
- The inner and outer surfaces of a 4-m × 10-m brick wall of thickness 20 cm are maintained at temperatures of 16°C and 4°C, respectively. If the rate of heat transfer through the wall is 1800 W, determine the rate of entropy generation within the wall.arrow_forwardTen grams of computer chips with a specific heat of 0.3 kJ/kg·K are initially at 20°C. These chips are cooled by placement in 5 grams of saturated liquid R-134a at –40°C. Presuming that the pressure remains constant while the chips are being cooled, determine the entropy change of the entire system. Is this process possible? Why?arrow_forwardtwo aluminum ingots, one weighing 1.5 kg at 450 degrees celsius while the other is 1.1 kg at 250 degrees celsius, are placed in an insulated enclosure. assuming there is no heat transfer from the ingots to the enclosure material, determine the final temperature and the entropy associated with the process.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





